Köhler and Milstein's Monoclonal Antibody Monstrosity (1975)
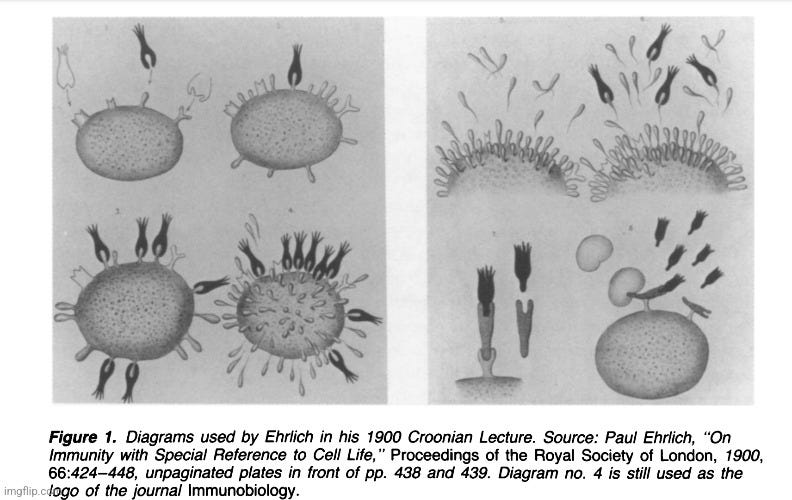
When I began my journey into the world of “antibodies,” I was rather shocked by the complete lack of direct scientific evidence that these entities actually existed and functioned as described. Like most, I assumed that what we were told about “antibodies” and the “immune” system was built upon solid scientific evidence. However, what I found was the exact opposite. Like the equally fictional “viruses,” the effects observed from unnatural processes created in a lab were construed as evidence for the existence of unseen entities that were somehow involved as the cause. In the case of the “antibody,” this began with the mixture and manipulation of blood from different species where the effects created were used as indirect evidence of existence. There was no purification (free on contaminants, host materials, pollutants, etc.) and isolation (separation from everything else) of these so-called “antibodies” at any point in time in order to study and experiment with. These lab-created effects were used by researchers to dream up the concept of an imaginary causal entity regarding how it looked, formed, and functioned. This whole process was instigated by the work of Robert Koch disciples Emil Von Behring and Paul Ehrlich. While Von Behring presented the lab-created effect, it was Ehrlich who presented the presumed cause when he laid out his side-chain theory of “antibody” production and his conceptual drawings of what French biologist Felix Le Dantec termed the “imaginary invalid” in the early 1900s.
As noted by American Pathologist Harry Gideon Wells on page 109 in his 1929 book The Chemical Aspects of Immunity, “antibodies” were only “known” due to altered reactivity of sera in a lab. They had no idea what “antibodies” were or if they even existed as material objects.
“We attribute this altered reactivity [of sera] to the presence of “antibodies,” despite the fact that we have absolutely no knowledge of what these antibodies may be, or even that they exist as material objects. Like the enzymes, we recognize them by what they do without discovering just what they are.”
The fact that effects were being applied to unknown entities was also noted in a 1910 German textbook Die Methoden der Immunodiagnostik und Immunotherapiewhich admitted that all attempts to isolate “antibodies” had failed. Only effects were known, and these effects were attributed to the unseen entities.
“In order to learn the nature of these antibodies attempts have been made to isolate them chemically. Thus far all such trials have been unsuccessful. It is even uncertain whether these so-called antibodies are definite chemical entities. Only the effects of the serum as a whole are known, and the ingredients in it to which these activities are attributed are thought of as antibodies.”
What proceeded were conceptual drawings by Ehrlich and others of entities that belonged to the imagination. Like “viruses,” the “antibodies” existed as part of what was known as the “domain of the invisible specimen behavior.” As noted by Henry Smith Williams and James Beveridge in the 1915 book The Mechanism of Immunization, “it would be absurd to imagine that the mechanical diagrams have any representation in the world of fact. They are figments of the imagination and may serve some useful purpose as picture books serve in teaching a child the alphabet.” According to the 1993 paper Ehrlich's "Beautiful Pictures" and the Controversial Beginnings of Immunological Imagery, the drawings were not seen as a “faithful image of reality,” and many felt that they “should be discarded because they were fundamentally misleading.” The paper went on to state that the material nature of the “antibody” was based upon faith, and that the status of the existence of these entities would remain uncertain until they were able to be properly purified.
“So, despite various professions of faith in the material nature of “antibodies,” their ontological status remained uncertain, a situation ascribed by some scientists to the failure to purify chemically the elusive entities and thus to ascertain whether they were indeed material substances. This, of course, begged the question, because in order to base an argument on the possible chemical purification of antibodies one had first to assume that antibodies were indeed discrete chemical substances, which is precisely what Ehrlich’s opponents contested.”
The authors noted that, to even ascertain whether “antibodies” were material substances, one had to beg the question and assume the existence of the “antibodies” as discrete chemical substances to begin with. This a form of logically fallacious thinking where the “argument's premises assume the truth of the conclusion, instead of supporting it. In other words, you assume without proof the stand/position, or a significant part of the stand, that is in question. Begging the question is also called arguing in a circle.” This pattern of logically fallacious thinking is often encountered in virology, and it is clearly no stranger to “antibody” research as well. Due to this inability to properly purify and isolate “antibodies,” the authors stated that this debate over the existence, nature, and-properties of “antibodies” lasted for decades. There were many competing theories and ideas tossed around as the researchers did not have the ability to find “antibodies” within the supposed natural environment in the serum of a host in order to study and manipulate during experimentation. The entire field of “antibody” research was vastly restricted by not being able to directly study the thing that they claimed to be studying. However, rather than attempting to resolve the issue by finding a way to obtain “antibodies” from the host in a purified and isolated state, the solution was to turn to the use of the cell culture techniques popularized by virologists in order to artificially create these unseen entities with the development of something that became known as the hybridoma technology. Presented below is how the pseudoscientific cell culture technology was called upon once again in 1975 in order to create the evidence for the existence of a conceptual entity never observed within its natural host environment.
“No, there is no such thing as a monoclonal antibody that, because it is monoclonal, recognizes only one protein or only one virus. It will bind to any protein having the same (or a very similar) sequence.”
-Clifford Saper, one of the world’s leading authorities on monoclonal antibodies, Harvard Medical School professor
https://off-guardian.org/2021/03/06/the-antibody-deception/
“Monoclonal antibodies are laboratory-made proteins that mimic the immune system’sability to fight off harmful pathogens such as viruses.”
https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19-0
By 1975, nearly a century after Emil Von Behring hypothesized the existence of unseen substances in the blood in 1890, there was still no direct proof that these substances, now known as “antibodies,” actually existed as claimed. There were plenty of hypotheses, theories, and indirect chemistry experiments accumulated over the numerous decades claiming to support this fictional concept, but the “antibody” as a whole, intact unit had never been purified and isolated directly from the fluids, visualized, and proven to function in the way that it was hypothesized via evidence generated from the scientific method. In fact, the inability to purify and isolate single “antibodies” from the billions said to be produced by the body was a key issue that hindered the entire field. Interestingly, the solution was not to find a way to actually purify and isolate “antibodies” so that these entities could be proven to really exist in order to have them on hand for experimentation. Rather, what the immunologists did was turn to the same cell culture tricks utilized by virologists in order to attempt to create “hybrids,” referred to as hybridomas, as a stand-in for the “real thing.” This involved the completely relevant to nature (note sarcasm) process where mouse cancer cells were claimed to be fused to mouse spleen cells from mice that had been injected with sheep blood:
The birth of monoclonal antibodies
The story of monoclonal antibodies began with the arrival of the Argentinean émigré César Milstein at the Medical Research Council Laboratory of Molecular Biology, Cambridge, UK, in 1963. Encouraged by Fred Sanger, who at the time was head of the Protein and Nucleic Acid Chemistry Division at the Laboratory of Molecular Biology, Milstein began investigating how antibody diversity is generated (Milestone 10). A key issue that was restricting the whole field at the time was the inability to isolate and purify single antibodies of known specificity from the billions produced in the body.
Henry Kunkel, based at the Rockefeller Institute in New York, had noted in 1951 that myeloma cells produce antibodies of just a single specificity; therefore, myeloma cell lines had become an important tool in antibody research. Dick Cotton, an Australian postdoctoral scientist new to the Milstein laboratory, showed in 1973 that the fusion of two immunoglobulin-producing myeloma cell lines produced a hybrid cell line that secreted myeloma antibodies of both parental types. Although the specificity of the antibodies produced was not known, this study was important for future work, as it described a technique for the generation of hybrid clones.
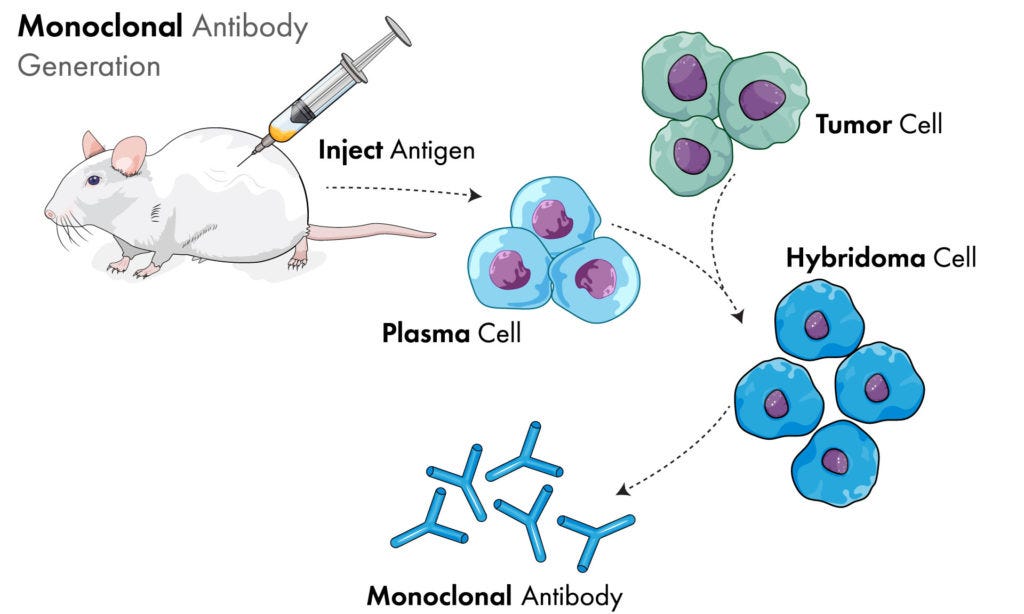
While presenting that work at the Basel Institute of Immunology, Milstein met the German Ph.D. student Georges Köhler, who joined Milstein's laboratory as a postgraduate in April 1974. Although several methods for cloning B cells that produce single antibodies of known specificity had been described—in particular, a technique developed by Norman Klinman at the University of Pennsylvania—these approaches had several limitations, including low antibody yield and short cell lifespan.Köhler and Milstein's solution was to generate a hybridoma. With the help of the laboratory technician Shirley Howe, they fused cells from the myeloma cell line P3-X63-Ag8 (which Cotton had been working on) with spleen cells from a mouse immunized with sheep red blood cells. The cells were then incubated in hypoxanthine aminopterin thymidine medium for up to 2 weeks; myeloma cells lack expression of the enzyme hypoxanthine guanine phosphoribosyltransferase, which is necessary for cell growth in this medium, and unfused B cells have a short lifespan. Therefore, only the fused cells survive. In this way they generated a hybridoma with the immortality of the myeloma cell and the ability to produce antibodies specific for sheep red blood cells, which originated from the spleen cell. After overcoming some technical difficulties, they successfully generated several hybridomas that secreted very large quantities of monoclonal antibodies.
In the Nature paper describing their discovery, Köhler and Milstein hinted at the potential implications of their findings, stating that “[s]uch cultures could be valuable for medical and industrial use.” However, the importance of the paper was largely overlooked at the journal, and it was omitted from the section reserved for findings of leading importance. It took a couple of years for the broader implications of the discovery to be realized, but with the realization of the potential advantages of the technique, as outlined in an editorial in the Lancet in June 1977 claiming the discovery could “have profound implications for medical practice”, Milstein soon became inundated with requests for cell samples. This paved the way for the early commercialization of monoclonal antibody technology.
The contributions made by Köhler and Milstein to scientific research were formally recognized when they were presented with both the Albert Lasker Basic Medical Research Award and the Nobel Prize in physiology or medicine, jointly with Niels Kaj Jerne, in 1984.
https://www.nature.com/articles/ni.3608
As if it really needed to be pointed out, immunology had most assuredly crossed into mad (pseudo)scientist territory by the mid-1970s (as if it hadn't already). Logically, if “antibodies” exist, the researchers should be able to properly purifiy and isolate these entities directly from the blood, especially in those who are assumed to have high amounts of “antibodies” in their blood either through “immunization” or as a byproduct of natural “infections.” Remember, there are supposedly billions of these entities produced within the body. It should then be able to be shown what these “antibodies” look like as well as to demonstrate if they truly offer any sort of protective effects through experimentation via the scientific method. It should not be necessary to artificially create and grow “antibodies” by mixing cancer cells and spleen cells with a HAT medium consisting of the synthetic chemical’s hypoxanthine, aminopterin, and thymidine along with fetal cow serum, antibiotics, and other chemical additives in a cell culture. Yet this is exactly what was done. It began when the work of Georges Köhler, who it is claimed was able to create cancerous forms of “antibody-producing” cells of unknown specificity, was fused with the work of Cesar Milstein, who it is claimed was able to make “antibody-producing” cells with known specificity that did not survive long. The result of this pseudoscientific marriage was the birth of the monstrosity known as the “monoclonal antibody.”
Kohler and Milstein's hybridoma technology (1975)
Our B cells, a type of white blood cell, can produce millions of different antibodies. However, each cell on its own can only produce antibodies of a certain, predetermined specificity, which means that many, many different B cells are needed to generate the multitude of antibodies needed by a healthy immune system. When the body is exposed to a given foreign antigen, it can cause the stimulation of a B cell that has fortuitously been endowed with the capacity to identify this particular antigen. The result is that this B cell then starts to divide to form a clone of cells which produce identical antibodies, or “monoclonal” antibodies.
This is how nature works. But in 1975George Kohler and Cesar Milstein, working at the MRC Laboratory of Molecular Biology in Cambridge, found a way of mimicking the effect to produce monoclonal antibodies “to order”. They did it by merging myeloma cells – cancerous cells resulting from the uncontrolled cells division resulting from a lymphocyte dividing to form a clone of identical cells – with antibody-producing B cells. By fusing the B cell with the myeloma cell, it acquires the ability to divide rapidly, allowing large numbers of identical antibody producing cells to be grown in cell culture.
The system is known as hybridoma technology because it involves cell hybrids to produce sets of identical monoclonal antibodies directed against specific antigens. Kohler and Milstein started out working independently. Milstein had developed cancerous forms of antibody-producing cells that grew and multiplied forever but which churned out antibodies of unknown specificity, while Kohler managed to get antibody-producing cells to make specific antibodies, but these cells didn’t survive for very long. By combining their discoveries, they came up with a way of making monoclonal antibodies of exquisite precision from cells that divided and divided and effectively lived forever.
Monoclonal antibodies, or “MAbs”, have revolutionised immunology in terms of analytic tests and diagnostics, and are now a standard treatment for certain forms of cancer with drugs such as Herceptin (trastuzumab), Avastin (bevacizumab) and Campath (alemtuzumab).
Kohler and Milstein were awarded a share of the 1984 Nobel prize in physiology or medicine for their breakthrough.
https://www.immunology.org/kohler-and-milsteins-hybridoma-technology-1975
The same unnatural cell culture tricks that were substituted as “proof” for the existence of “viruses” beginning in 1954 were now being applied to “antibodies.” The men behind this transition, Georges Köhler and Cesar Milstein, were awarded the Nobel Prize in 1984 for developing the hybridoma technique since scientists were unable to efficiently produce “specific antibodies” at the time. The Nobel write-up stated that the award was given “for theories concerning the specificity in development and control of the immune system and the discovery of the principle for production of monoclonal antibodies.” In other words, they were awarded for adding their own ideas on specificity and control of the “immune” system to the ever-changing narrative as well as for coming up with a way to artificially create these unseen substances in the lab.
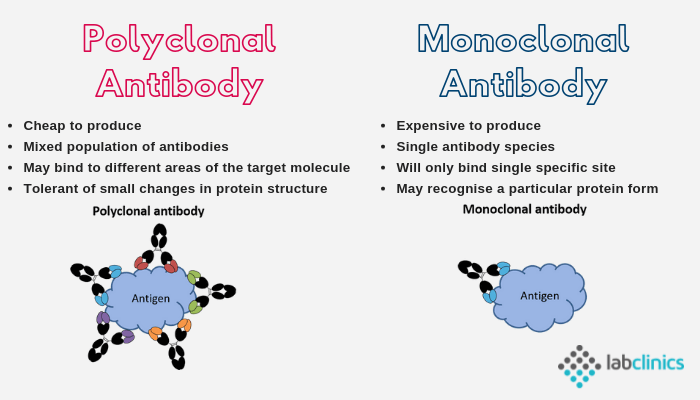
Was this really an invention that was worthy of such a “prestigious” prize? The biggest claim to fame for Köhler and Milstein's “monoclonal antibodies,” beyond the assertion that only one type of '“antibody” is present in a mixture, is their supposed specificity over the regularly produced “polyclonal antibodies” that are said to be made up of many different types. This means that the artificially created “antibodies” are supposed to be better at recognizing the right targets than those claimed to be “found in nature.” However, it has been known for quite some time that “monoclonal antibodies” are not specific, with admittances that they regularly bind to unwanted targets. In fact, as noted by the opening quote from Harvard Medical School professor Clifford Saper, one of the world’s leading authorities on “monoclonal antibodies,” there is no such thing as a “monoclonal antibody” that recognizes only one protein or only one “virus” as it will bind to any protein having the same (or a very similar) sequence. A study from 2018 found that “monoclonal antibodies” are, in many cases, not even “monoclonal,” with results showing that 31.9% of hybridomas producing “monoclonal antibodies” actually secrete multiple “antibody species.” According to an article describing the results, this cocktail of “antibody species” has resulted in reduced binding to the intended target as well as binding to unintended targets. Another finding was that the relative abundance of the “antibody species” varies between batches, making it difficult to obtain reproducible results. The assertion that 'monoclonal antibody' samples contain multiple 'species' is an ingenious way to explain away contradictory evidence. However, the reproducibility issues with 'antibodies' have been known since at least 2015. It has led to a reproducibility crisis where many “scientific” papers have been considered to be false due to the unreliable, unreproducible, and irreplicable results from the utilization of these lab-created concoctions.
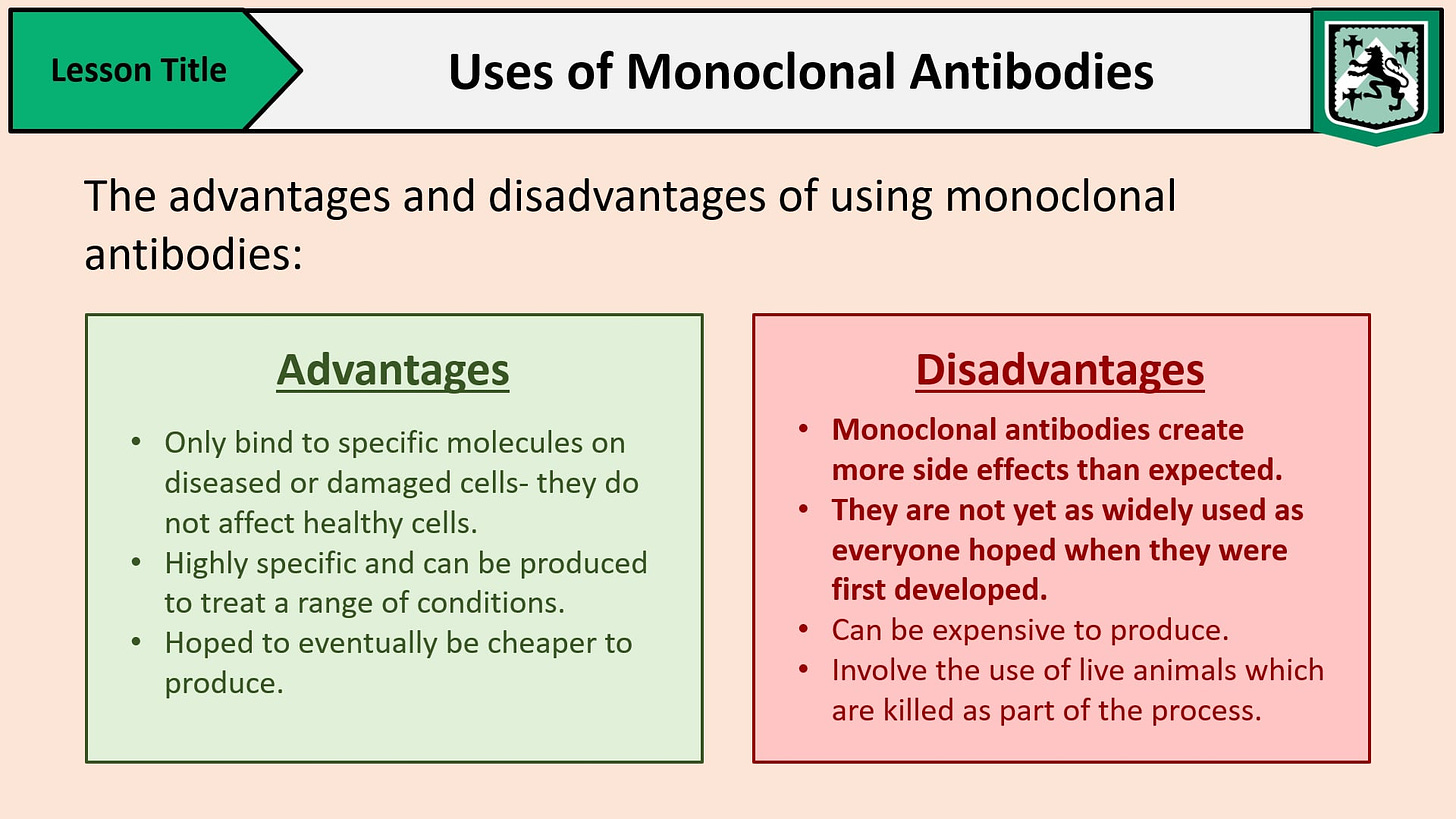
Another supposed benefit stemming from the discovery of this process is that these man-made cancer/animal hybrid creations are used as “monoclonal antibody therapies” in order to prevent severe disease. While the creation of these artificial “antibodies” has been heralded as a breakthrough for their role in testing and treatment for “infectious” diseases as well as supposedly reducing drug toxicity and suppressing rejection in organ transplants, it should come as no surprise that these mouse cancer/spleen cell hybrids carry various admitted side-effects and risks from their use inside humans. Known dangerous reactions include developing acute anaphylaxis, serum sickness, autoimmune disease, “infections” and cancers, organ-specific adverse events, and, for some strange reason, the generation of “antibodies” themselves:
The safety and side effects of monoclonal antibodies
“Monoclonal antibodies (mAbs) are now established as targeted therapies for malignancies, transplant rejection, autoimmune and infectious diseases, as well as a range of new indications. However, administration of mAbs carries the risk of immune reactions such as acute anaphylaxis, serum sickness and the generation of antibodies. In addition, there are numerous adverse effects of mAbs that are related to their specific targets, including infections and cancer, autoimmune disease, and organ-specific adverse events such as cardiotoxicity. In March 2006, a life-threatening cytokine release syndrome occurred during a first-in-human study with TGN1412 (a CD28-specific superagonist mAb), resulting in a range of recommendations to improve the safety of initial human clinical studies with mAbs. Here, we review some of the adverse effects encountered with mAb therapies, and discuss advances in preclinical testing and antibody technology aimed at minimizing the risk of these events.”
https://pubmed.ncbi.nlm.nih.gov/20305665/
Sounds “safe and effective,” just like vaccines (note sarcasm again). In April 2022, I examined “monoclonal antibody therapies” and the similarities that they share with snake anti-venom in the article Beware the Snake Oil Salesmen. This included looking at how these therapies are made, their admitted ineffectiveness, and the similar toxic effects from the treatments. The evidence presented in the article was in regard to the use of “monoclonal antibodies” for “Covid-19,” and it demonstrated that these therapies were not only ineffective, but dangerous. For example, according to a September 2021 Cochrane review of the available studies, they found insufficient evidence to claim that “monoclonal antibodies” are an effective treatment for “SARS-COV-2:”
Are laboratory-made, COVID-19-specific monoclonal antibodies an effective treatment for COVID-19?
“The evidence for each comparison is based on single studies. None of these measured quality of life. Our certainty in the evidence for all non-hospitalised individuals is low, and for hospitalised individuals is very low to moderate. We consider the current evidence insufficient to draw meaningful conclusions regarding treatment with SARS-CoV-2-neutralising mAbs.”
In other words, the evidence for the usefulness of “monoclonal antibodies” is non-existent. Unfortunately, the Cochrane Review failed to point out that there are various risks and adverse reactions associated with their use:
Do mAbs have risks?
“Therapeutic mAbs, typically administered by intravenous (IV) infusion, have been a valuable and generally safe treatment option for a variety of conditions for many years. However, they are also known to cause a range of side effects and reactions, which can be immediate or delayed. Serious adverse events associated with mAbs include infusion reactions, acute anaphylaxis, and serum sickness, as well as longer-term complications such as infections, cancer, autoimmune disease, and cardiotoxicity.”
https://www.ecri.org/components/PPRM/Pages/QAMonoclonalAntibodyCOVID19.aspx?PF=1&source=print
Due to this ineffectiveness as well as the dangerous effects associated with this therapy, the FDA pulled the Emergency Use Authorization (EUA) for several “monoclonal antibodies” as it was determined that the various risks involved with using these treatments outweighed the unproven “benefits.” Of course, there was the built-in excuse for the ineffectiveness of the treatments. In this case, the story revolved around the supposed mutation of the crafty “virus” and its evasion from the “protective” effects of the “antibodies.” In other words, it wasn't that the treatment was ineffective and dangerous, it was that the “virus” changed clothes and successfully disguised itself once again, tricking the “antibody” defenders and keeping them from being able to provide protection from severe disease. The recalled “mAbs” were then replaced with “updated versions.”
Can monoclonal antibodies still be used to treat COVID-19?
“Because each type of monoclonal antibody is carefully designed to work against a specific protein on the COVID-19 virus, a mutation in that viral protein could render the monoclonal antibody ineffective. Indeed, this is exactly what happened as different COVID-19 variants have evolved. In fact, the FDA has restricted or even withdrawn their emergency use authorization of some monoclonal antibodies that are not effective against the omicron variant.”
https://health.osu.edu/health/virus-and-infection/monoclonal-antibodies-still-treat-covid-19
Thus, it can be seen that, in regard to the claims made about the usefulness of the artificially created “monoclonal antibodies,” they are rather inaccurate as these cell culture concoctions are regularly not specific, are not of one type, lead to unreproducible results, and can cause severe disease and death when utilized as a treatment. Thus, it should be asked, what “breakthrough benefits” were provided from Köhler and Milstein's “discovery” that deserved awarding them with a Nobel Prize?
This evidence should be enough to provoke anyone to reconsider the claims made about the benefits and usefulness of “monoclonal antibodies.” However, to further highlight the absurdity of these entities, let's take a look at the pseudoscientific methods from the paper that resulted in their “discovery” in order to seal the deal. Presented below is Köhler and Milstein's Nobel Prize-winning paper from 1975 for your viewing pleasure. When reading this paper, ask yourself how the experiments performed reflect nature in any way, shape, or form. What is the hypothesis that was based upon an observed natural phenomenon? What was the independent variable (presumed cause) in the experiment, and what was the dependent variable (effect being studied)? Were proper controls performed? Keep in mind that none of the tests used to detect and measure these so-called “antibodies” in this study were ever calibrated and validated to purified and isolated “antibodies” taken directly from the blood. These entities are assumed to be present based upon indirect evidence. There are zero electron micrographs demonstrating any purified and isolated “monoclonal antibodies,” which one would assume would be easy enough to obtain from a product claimed to be made up of nothing but the Y-shaped particles. Regardless, I will leave it up to the reader to decide what scientific value this paper holds and whether or not it should serve as evidence for the existence of these entirely hypothetical entities.
Continuous cultures of fused cells secreting antibody of predefined specificity
THE manufacture of predefined specific antibodies by means of permanent tissue culture cell lines is of general interest. There are at present a considerable number of permanent cultures of myeloma cellsand screening procedures have been used to reveal antibody activity in some of them. This, however, is not a satisfactory source of monoclonal antibodies of predefined specificity. We describe here the derivation of a number of tissue culture cell lines which secrete anti-sheep red blood cell (SRBC) antibodies. The cell lines are made by fusion of a mouse myeloma and mouse spleen cells from an immunised donor. To understand the expression and interactions of the Ig chains from the parental lines, fusion experiments between two known mouse myeloma lines were carried out.
Each immunoglobulin chain results from the integrated expression of one of several V and C genes coding respectively for its variable and constant sections. Each cell expresses only one of the two possible alleles (allelic exclusion; reviewed in ref. 3). When two antibody-producing cells are fused, the products of both parental lines are expressed, and although the light and heavy chains of both parental lines are randomly joined, no evidence of scrambling of V and C sections is observed. These results, obtained in an heterologous system involving cells of rat and mouse origin, have now been confirmed by fusing two myeloma cells of the same mouse strain, and provide the background for the derivation and understanding of antibody-secreting hybrid lines in which one of the parental cells is an antibody-producing spleen cell.
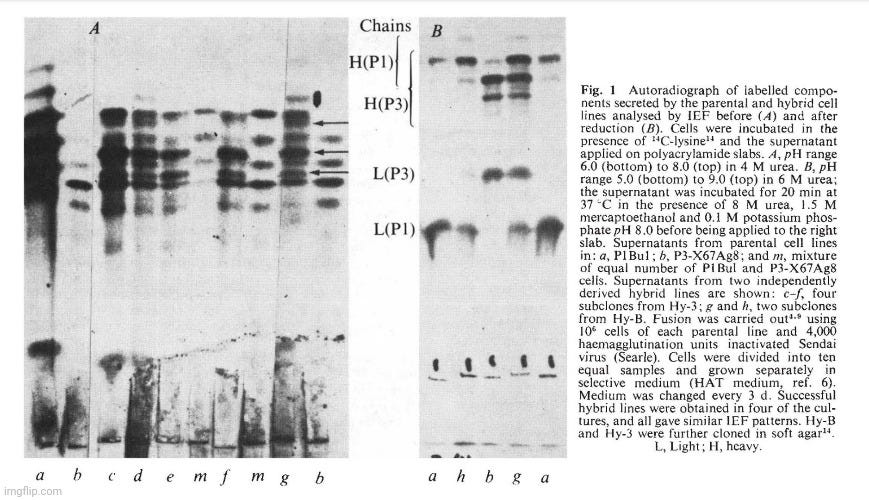
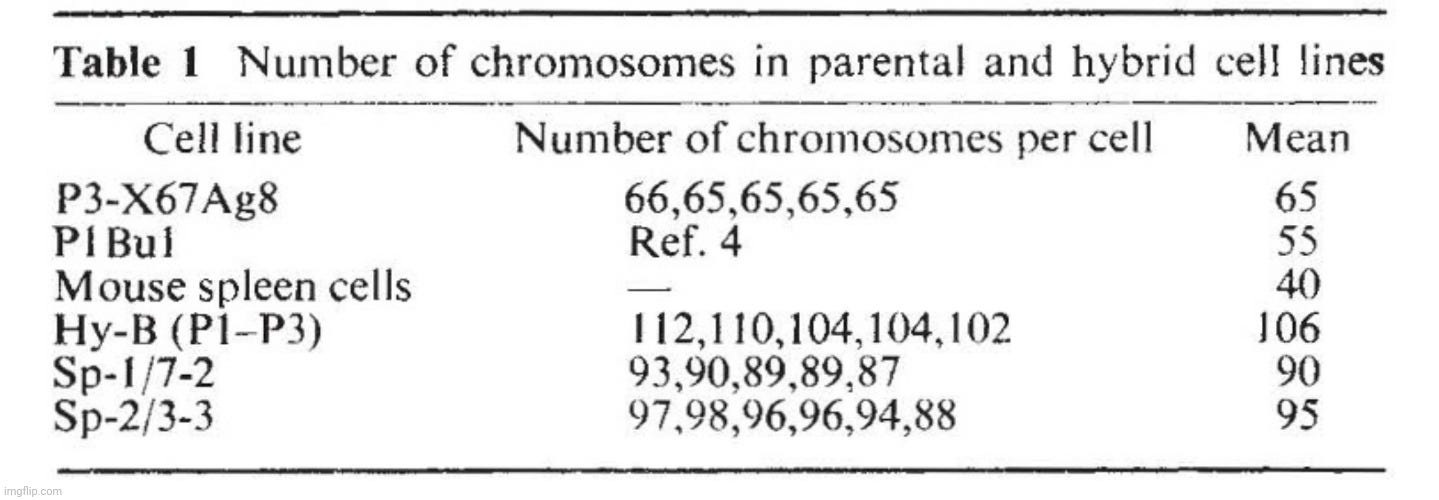
Two myeloma cell lines of BALB/c origin were used. PI Bul is resistant to 5-bromo-2'-deoxyuridine does not grow in selective medium (HAT, ref. 6) and secretes a myeloma protein, Adj PC5, which is an lgG2A (K), (ref. 1). Synthesis is not balanced and free light chains are also secreted. The second cell line, P3-X63Ag8, prepared from P3 cclls 2 , is resistant to 20 11g ml-1 8-azaguaninc and does not grow in HAT medium. The protein secreted (MOPC 21) is an lgG I (K) which has been fully sequenced. Equal numbers of cells from each parental line were fused using inactivated Sendai virus and samples containing 2 x 105 cells were grown in selective medium in separate dishes. Four out of ten dishes showed growth in selective medium and these were taken as independent hybrid lines, probably derived from single fusion events. The karyotype of the hybrid cells after 5 months in culture was just under the sum of the two parental lines (Table I). Figure I shows the isoelectric focusing (IEF) pattern of the secreted products of different lines. The hybrid cells (samples c-h in Fig. 1) give a much more complex pattern than either parent (a and b) or a mixture of the parental lines (m). The important feature of the new pattern is the presence of extra bands (Fig. I, arrows). These new bands, however, do not seem to be the result of differences in primary structure; this is indicated by the IEF pattern of the products after reduction to separate the heavy and light chains (Fig. I B). The IEF pattern of chains of the hybrid clones (Fig. I B, g) is equivalent to the sum of the IEF pattern (a and b) of chains of the parental clones with no evidence of extra products. We conclude that, as previously shown with interspecies hybrids, new lg molecules are produced as a result of mixed association between heavy and light chains from the two parents. This process is intracellular as a mixed cell population does not give rise to such hybrid molecules (compare m and g, Fig. lA). The individual cells must therefore be able to express both isotypes. This result shows that in hybrid cells the expression of one isotype and idiotype does not exclude the expression of another: both heavy chain isotypes ( y1 and y2a) and both V11 and both VL regions (idiotypes) are expressed. There are no allotypic markers for the C K region to provide direct proof for the expression of both parental C K regions. But this is indicated by the phenotypic link between the V and C regions.
Figure I A shows that clones derived from different hybridisation experiments and from subclones of one line are indistinguishable. This has also been observed in other experiments (data not shown). Variants were, however, found in a survey of 100 subclones. The difference is often associated with changes in the ratios of the different chains and occasionally with the total disappearance of one or other of the chains. Such events are best visualised on IEF analysis of the separated chains (for example, Fig. lh, in which the heavy chain of P3 is no longer observed). The important point that no new chains are detected by IEF complements a previous study of a rat-mouse hybrid line in which scrambling of V and C regions from the light chains of rat and mouse was not observed. In this study, both light chains have identical C K regions and therefore scrambled VL-CL molecules would be undetected. On the other hand, the heavy chains are of different subclasses and we expect scrambled V H-C H to be detectable by IEF. They were not observed in the clones studied and if they occur must do so at a lower frequency. We conclude that in syngeneic cell hybrids (as well as in interspecies cell hybrids) V-C integration is not the result of cytoplasmic events. Integration as a result of DNA translocation or rearrangement during transcription is also suggested by the presence of integrated mRNA molecules 11 and by the existence of defective heavy chains in which a deletion of V and C sections seems to take place in already committed cells.
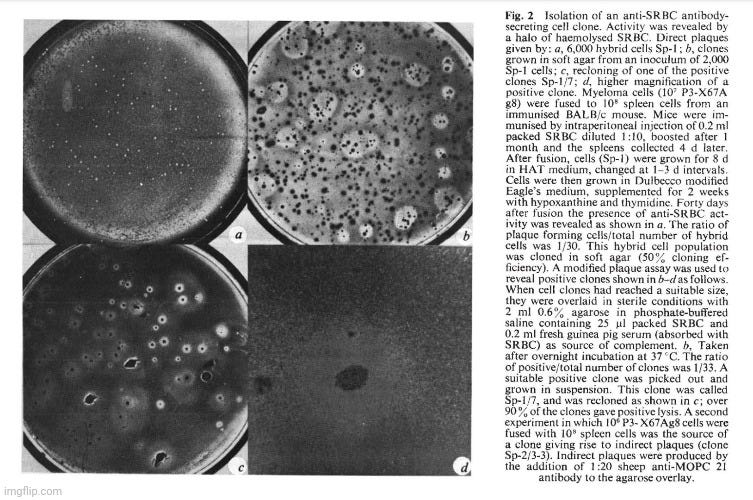
The cell line P3-X63Ag8 described above dies when exposed to HAT medium.Spleen cells from an immunised mouse also die in growth medium. When both cells are fused by Sendai virus and the resulting mixture is grown in HAT medium, surviving clones can be observed to grow and become established after a few weeks. We have used SRBC as immunogen, which enabled us, after culturing the fused lines, to determine the presence of specific antibody-producing cells by a plaque assay technique 13 (Fig. 2a). The hybrid cells were cloned in soft agar 14 and clones producing antibody were easily detected by an overlay of SRBC and complement (Fig. 2b). Individual clones were isolated and shown to retain their phenotype as almost all the clones of the derived purified line are capable of lysing SRBC (Fig. 2c). The clones were visible to the naked eye (for example, Fig. 2d). Both direct and indirect plaque assays 13 have been used to detect specific clones and representative clones of both types have been characterised and studied.
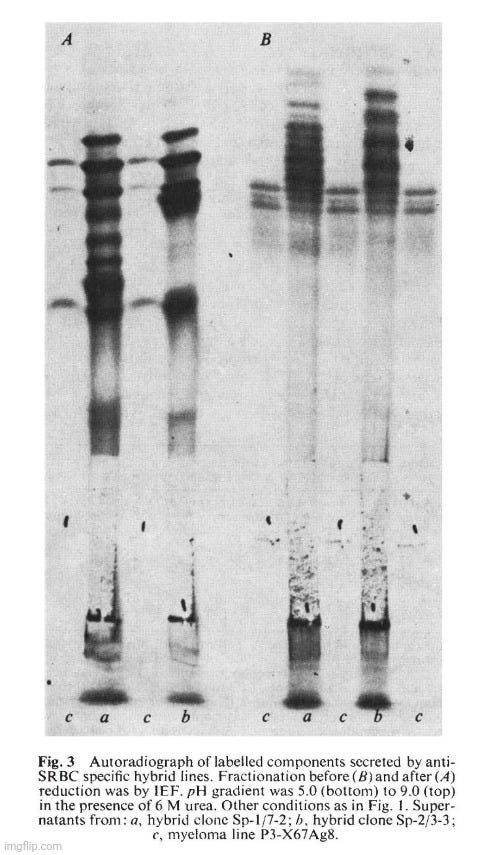
The derived lines (Sp hybrids) are hybrid cell lines for the following reasons. They grow in selective medium. Their karyotype after 4 months in culture (Table 1) is a little smaller than the sum of the two parental lines but more than twice the chromosome number of normal BALB/c cells, indicating that the lines are not the result of fusion between spleen cells. In addition the lines contain a metacentric chromosome also present in the parental P3-X67Ag8. Finally, the secreted immunoglobulins contain MOPC 21 protein in addition to new, unknown components. The latter presumably represent the chains derived from the specific anti-SRBC antibody. Figure 3A shows the IEF pattern of the material secreted by two such Sp hybrid clones. The IEF bands derived from the parental P3 line are visible in the pattern of the hybrid cells, although obscured by the presence of a number of new bands. The pattern is very complex, but the complexity of hybrids of this type is likely to result from the random recombination of chains (see above, Fig. 1). Indeed, IEF patterns of the reduced material secreted by the spleen-P3 hybrid clones gave a simpler pattern of lg chains. The heavy and light chains of the P3 parental line became prominent, and new bands were apparent.
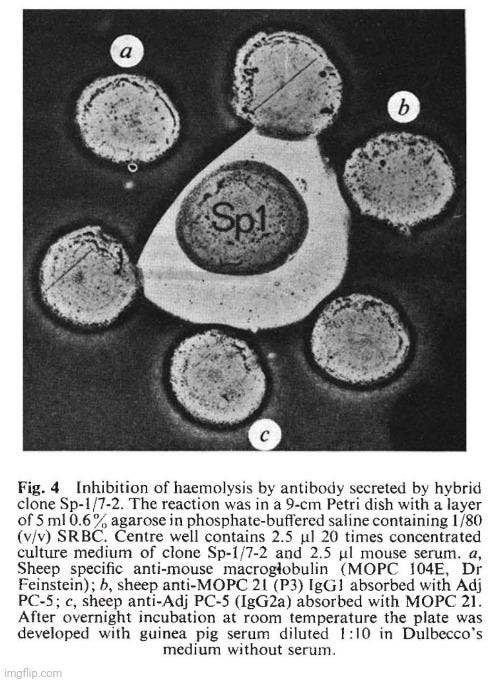
The hybrid Sp-1 gave direct plaques and this suggested that it produces an lgM antibody. This is confirmed in Fig. 4 which shows the inhibition of SRBC lysis by a specific anti-lgM. This is confirmed in Fig. 4 which shows the inhibition of SRBC lysis by a specific anti-lgM antibody. IEF techniques usually do not reveal 19S IgM molecules. IgM is therefore unlikely to be present in the unreduced sample a (Fig. 3B) but u chains should contribute to the pattern obtained after reduction (sample a, Fig. 3A).
The above results show that cell fusion techniques are a powerful tool to produce specific antibody directed against a predetermined antigen. lt further shows that it is possible to isolate hybrid lines producing different antibodies directed against the same antigen and carrying different effector functions (direct and indirect plaque).
The uncloned population of P3-spleen hybrid cells seems quite heterogeneous. Using suitable detection procedures it should be possible to isolate tissue culture cell lines making different classes of antibody. To facilitate our studies we have used a myeloma parental line which itself produced an lg. Variants in which one of the parental chains is no longer expressed seem fairly common in the case of Pl-P3 hybrids (Fig. lh). Therefore selection of lines in which only the specific antibody chains are expressed seems reasonably simple. Alternatively, non-producing variants f myeloma lines could be used for fusion.
We used SRBC as antigen. Three different fusion experiments were successful in producing a large number of antibody-producing cells. Three weeks after the initial fusion, 33/1,086 clones (3 %) were positive by the direct plaque assay.The cloning efficiency in the experiment was 50%. In another experiment, however, the proportion of positive clones was considerably lower (about 0.2 %). In a third experiment the hybrid population was studied by limiting dilution analysis. From 157 independent hybrids, as many aas15 had anti-SRBC activity. The proportion of positive over negative clones is remarkably high. It is possible that spleen cells which have been triggered during immunisation are particularly successful in giving rise to viable hybrids. It remains to be seen whether similar results can be obtained using other antigenes.
The cells used in this study are all of BALB/c origin and the hybrid clones can be injected into BALB/c mice to produce solid tumours and serum having anti-SRBC activity. It is possible to hybridise antibody-producing cells from different origins. Such cells can be grown in vitro in massive cultures to provide specific antibody.Such cultures could be valuable for medical and industrial use.
https://www.nature.com/articles/256495a0
https://doi.org/10.1038/256495a0
In Summary:
Köhler and Milstein's interest was in manufacturing predefined specific “antibodies” by means of permanent tissue culture cell lines
They stated that permanent myeloma (cancer) cell lines are available, and some have been screened for “antibody” activity, but they are not a good source of specific “monoclonal antibodies”
Thus, they decided to fuse mouse myeloma and mouse spleen cells from a mouse “immunized” with blood from a sheep
They state findings of “antibody-producing” cell fusion through a heterozygous system involving both rat and mice cells were confirmed by fusing mouse cells of the same strain
They fused parental cells from each strain with the Sendai “virus”
(Quick side note on the Sendai “virus” used to fuse these cells:
“Given the history, it is not surprising that many investigators have questioned the authenticity of the original reports of human infection with Sendai virus. The consensus today is that humans are not natural hosts of Sendai virus (Fukumi et al., 1954, 1959; Fukumi and Nishikawa, 1961; Chanock et al., 1963; Ishida and Homma, 1978); rather, the natural hosts of Sendai virus are exclusively laboratory rodents.”
https://www.sciencedirect.com/book/9780122625022/diseases
“In contrast, while there is no evidence that wild rodents are infected with sendai virus (SeV), and its natural host remains unknown, SeV causes serious outbreaks of disease in colonies of laboratory mice and rats.”
https://jvi.asm.org/content/73/4/3125
“Due to the low prevalence of clinical signs, diagnosis is best achieved by detection of antibodies to the virus and demonstration of typical lesions in the respiratory tract. The ELISA is the test of choice for diagnosis of Sendai virus infections in rats.”
https://www.sciencedirect.com/science/article/pii/B9780122639517500077
The Sendai “virus” is unknown in the wild and the natural hosts of this “virus” are not natural but LABORATORY rodents... 🤔
We also have circular reasoning where the “virus” is detected by “antibodies” due to a lack of symptoms. Thus, one fictional entity is being used to claim the presence of the other, and vice versa. 🤷♂️)
4 out of 10 cultures showed "growth" and were considered hybrid lines PROBABLYderived from single fusion events
The cells were cultured for 5 months
They used Isoelectric Focusing to determine pattern similarity between parental and hybrid cell lines
(A quick side note on Isoelectric Focusing:
WHAT IS ISOELECTRIC FOCUSING?
Isoelectric focusing (IEF) is a powerful analytical tool for the separation of proteins. In order to ensure the high performance of analysis, isoelectric point (pI) standards are needed. In addition to classical protein based standards, low molecular weight compounds have been developed and successfully examined in capillary IEF and IEF-gel electrophoresis. Gel electrophoresis, the common technology for IEF, minimizes convection and introduces an additional gel-sieving effect to separate proteins by size. However, it has several disadvantages such as lengthy analysis time, limited resolution, and difficulty in detection.These challenges have been largely overcome by the development of IPG-strips (immobilized pH gradients) for use in high-resolution pI-separation.”
"One of the most critical issues in isoelectric focusing, whether in capillary electrophoresis or slab gel, is standardization of results. For that purpose typical protein standards have been used which could show certain disadvantages:
Limited stability of solutions
Inadequate purity for application as a standard
Lot-to-lot inconsistency
https://www.sigmaaldrich.com/technical-documents/articles/biofiles/isoelectric-focusing.html
As can be seen, isoelectric focusing is an indirect method for separating proteins that carries many drawbacks. It is incapable of saying what is actually in the mixture or whether only one type of protein is present.)
There were no allyotopic markers for the C K region to provide direct proof for the expression of both parental C K regions
They claim that the clones are indistinguishable from parental lines in data not shown, but then state that variants were found in a survey of 100 clones
Integration as a result of DNA translocation or rearrangement during transcription was suggested by the presence of integrated mRNA molecules and by the existence of defective heavy chains in which a deletion of V and C sections seemed to take place in already committed cells
Both cell lines alone die in their respective mediums but "survive" when fused together with the Sendai "virus" and grown in HAT medium
Plaque assay was used to indirectlydetermine “antibody-producing” cells after they were cultured
They claim the fused cells are hybrid due to growing in their selective medium and their karyotype (the number and visual appearance of the chromosomes in the cell nuclei of an organism or species) is a little smaller than the sum of the two parental lines
The fused lines were said to contain new, unknown components
They presume that these unknown components represent the chains for anti-SRBC antibodies
The IEF pattern was said to be complex, and they assume this is likely due to random recombination of chains
The hybrid gave direct plaques which suggested it produces an IgM “antibody”
They admit IEF does not usually reveal IgM molecules, thus it is unlikely to be in the unreduced sample
They claim that is should be possible to isolate tissue culture cell lines to produce different classes of “antibodies”
Three weeks after fusion, only 3%(33/1,086) of clones were “positive” after plaque assay
Only 15 out of 157 had anti-SRBC activity
The researchers stated that it remained to be seen if similar results could be obtained from other antigens
The hybrid clones used in the study could be injected into mice to produce tumors
They claimed it is now possible to hybridize “antibody-producing” cells in vitro (in the lab outside of living organisms) in massive cell cultures
Cesar Milstein wrote about the hybridoma technology and why he got involved in immunology when accepting the Nobel Prize in 1984. In his paper From the Structure of Antibodies to the Diversification of the Immune Response, he stated that what had attracted him to immunology “was that the whole thing seemed to revolve around a very simple experiment: take two different antibody molecules and compare their primary sequences,” noting that the “secret of antibody diversity would emerge from that.” Milstein pointed out that, at the time, it was fortunate that he was “sufficiently ignorant of the subject not to realize how naive I was being.” Clearly, with it being impossible to purify and isolate single “antibodies” from the billions said to be produced by the body, his “simple experiment” was not so simple.
To overcome this problem, the (pseudoscientific) tissue culture methods were introduced to his laboratory which “had a major impact on the direction of our research.” However, upon working with myeloma cells, Milstein stated that, of the myeloma cells available, “none proved suitable in our hands.” Regardless, Milstein recounted that “in a funny way our lack of success led to our breakthrough; because, since we could not get a cell line off the shelf doing what we wanted, we were forced to construct it.” In other words, Milstein and Köhler were unable to achieve results utilizing the already suspect tissue culture experiments with regular myeloma cells, so they had to go about and fabricate a method to create hybrids using the mouse cancer cells with the Sendai “virus” and the mouse spleen cells, as discussed in their foundational paper, in order to get the results that they wanted to see.
Nonetheless, after “early successes,” Milstein admitted that they “ran into technical difficulties and could not get our fusion experiments to work for quite some time.” It wasn't until Giovanni Galfré, a recruit who later joined the team, “discovered that one of our stock solutions had become contaminated with a toxic substance” that “an improved reliable protocol was developed.” This excerpt highlights one of the key ways of explaining away failures to replicate results utilizing the contamination excuse. It is always left to “improving” the technology or protocols in the future, and once those results are shown to be unreliable, there will be future “improvements” to overcome the new contradictions, never questioning the fraudulent foundation upon which the original results were built.
Milstein later pointed out that, when an animal encounters an antigen, “different antibodies are secreted and mixed in the serum.” As the individual “antibody molecules” are extremely similar, “once mixed they cannot be separated from each other.” Milstein admitted that it was impossible to study the diversity of the “antibody” response to a given immunogen until the advent of his hybridoma technology. Thus, it was not possible to truly study “antibodies” as “it has been known for a long time that even the simplest antigenic determinants are recognized by an unknown variety of antibody molecules.” According to Milstein, with the creation of the artificial “monoclonal antibodies,” the old questions of how complex the collection of “antibody molecules” produced by the animal as a response to a particular antigen is, and how the individual molecules differ from each other, could finally be answered. In other words, artificially created entities were supposed to be able to tell us something about unobservable processes occurring in nature. Oblivious to how ridiculous this notion truly is, Milstein proudly proclaimed that his invention could answer the question of “how, in real life in the animal, are all those genetic events capable of producing antibody diversity actually operate in response to an antigenic stimulus?” He stated that “the immunochemistry of the future will revert to an instructional approach where the antigen will tell us what antibody structure we should construct.” Perhaps realizing the unintentional irony, Milstein stated, “Although this is not science fiction, we need to overcome the theoretical problems involved in the translation of one-dimensional reality into a valid three-dimensional prediction.” Milstein admitted that the “success” of the hybridoma technology was “to a large extent the result of unexpected and unpredictable properties of the method.” It was research that was not “considered commercially worthwhile, or of immediate medical relevance” and that it “resulted from esoteric speculations, for curiosity’s sake, only motivated by a desire to understand nature.”
However, unlike how Milstein wanted to portray his work, it is clear that there was nothing close to reality occurring within their cell culture experiments, which were a monstrous mixture of cancer and spleen cells, antibiotics, cow serum, and various synthetic chemical additives that would never intermingle unless brought together in a lab. There was no information derived from their experiments that could help lead to an understanding of what actually happens in nature. The only “theories” derived were pseudoscientific ones rooted entirely in the science fiction section. As had happened with virology, the horrific cell culture technique reared its ugly head to be used as a proof of concept, this time in the case of “monoclonal antibodies.” Is it any wonder why these grotesque unnatural cultures produce such sickness in humans injected with them in the form of “monoclonal antibody” therapy? There is nothing natural about the Frankenstein methods used in these studies. The results from the methods utilized by Köhler and Milstein are definitely not proof of the existence of the unseen hypothesized substances. All that can be gleaned from these experiments is that creating a witches brew in a lab led to unproven claims that resulted in the use of injecting these concoctions directly into humans, which has been shown to create sickness and disease rather than health and “immunity.” In other words, the direct opposite of the proposed function and intended purpose of these yet to be proven entities.
Dr Sam Bailey examined Toxic Shock Syndrome in relation to both the germ and the terrain.
The Baileys also offered another insightful Q&A session.
Dr. Mark Bailey supplied another brilliant short essay titled Virus, Bacteriophage & Single “Virus” Genomics that was released as a video as well.
Christine Massey FOIs offered up more Freedom of Information Requests focused on the “avian flu.”
Dawn Lester put the spotlight on the fear propaganda within the mainstream media attempts to trick us into believing that there is another “Covid summer wave.”
Betsy looked at the appeal to authority logical fallacy and how important it is to have the ability to think and trust oneself over “experts.”
Aldhissla had a slew of excellent content, starting with a look at how “uninfected” cell cultures regularly showed the same cytopathogenic effects attributed to “viruses.”
The lack of “infectiousness” and “contagiousness” of mumps, chickenpox, and smallpox were explored as well.





No comments:
Post a Comment