Report 67: Part 4b – Rhabdomyolysis – a.k.a., “Jellied Muscle” – After mRNA Gene Therapy Injections
April 14, 2023 • by Robert W. Chandler, MD, MBA
This report is Part 4b in a series. It follows Part 1, “Report 56: Autopsies Reveal Medical Atrocities of Genetic Therapies Being Used Against a Respiratory Virus;” Part 2, “Report 58: Part 2 – “Autopsies Reveal Medical Atrocities of Genetic Therapies Being Used Against a Respiratory Virus;” and Part 3, “Report 61: Part 3 –Ute Krüger, MD, Breast Cancer Specialist, Reveals Increase in Cancers and Occurrences of ‘Turbo Cancers’ Following Genetic Therapy ‘Vaccines’,” and “Report 64: Part 4a – Re-Humanizing Data Using ‘Intramyocardial Inflammation after COVID-19 Vaccination: An Endomyocardial Biopsy-Proven Case Series.“
I. Definition: What is Rhabdomyolysis?
Rhabdomyolysis is a process involving the catastrophic breakdown of skeletal or striated muscle with release of muscle components that have a toxic physiologic effect on the whole person and can be difficult to manage medically. A florid case often has a fatal outcome.
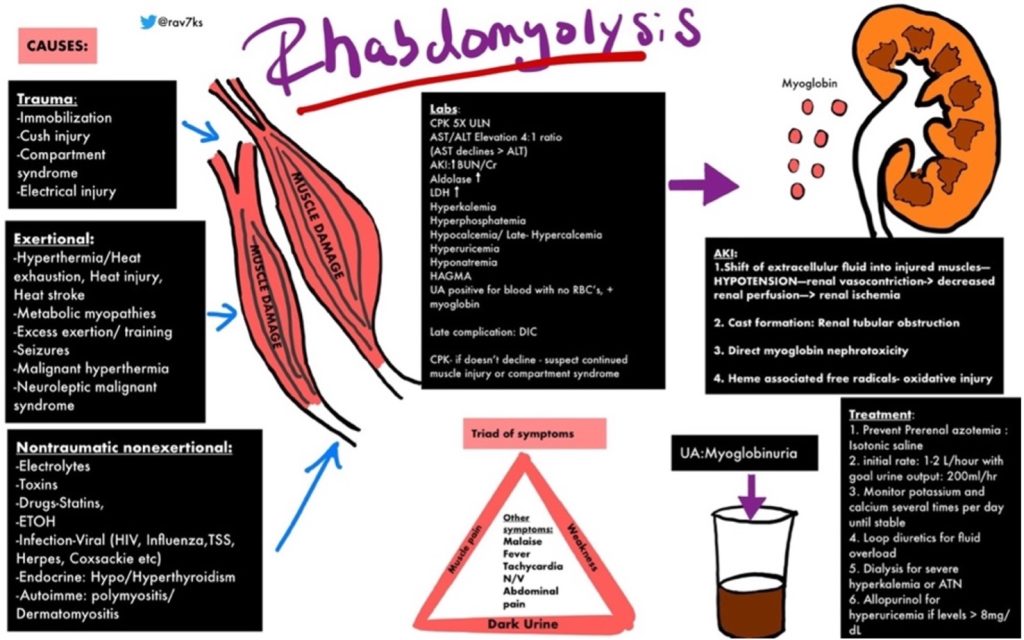
https://img.grepmed.com/uploads/9596/diagnosis-treatment-causes-rhabdomyolysis-differential-original.jpeg (Used with permission by the creator, Dr. Rav Singh K.)
Dr. Singh’s graphic illustrates the complexity of rhabdomyolysis. This chart will make more sense if the reader reviews the chart briefly, then reads the information to follow, and then looks at the graphic a second time.
Bhai and Dimachkie have published a worthwhile review of this condition. https://www.uptodate.com/contents/rhabdomyolysis-clinical-manifestations-and-diagnosis
The following is from their review:
- Symptoms and signs — Rhabdomyolysis is characterized clinically by the triad of myalgias, muscle weakness, and red to brown urine due to myoglobinuria. Biochemically, several serum muscle enzymes are elevated, including CK. The degree of muscle pain and other symptoms varies widely.
- Most of the symptoms of rhabdomyolysis are nonspecific.
- Classic triad — The characteristic triad of complaints in rhabdomyolysis is muscle pain, weakness, and dark urine. However, the full triad is observed in only 1 to 10 percent of cases.
- Muscle — When present, muscle symptoms of rhabdomyolysis may develop over hours to days.
- Pain – In hospitalized patients with rhabdomyolysis, muscle pain affects 23 to 80 percent of patients. Muscle pain, when present, is typically most prominent in proximal muscle groups, such as the thighs and shoulders, and in the lower back and calves [2,5]. Other muscle symptoms include stiffness and cramping.
- Weakness – Muscle weakness may be present depending upon the severity of muscle injury and affects 12 to 70 percent of hospitalized patients with rhabdomyolysis. Weakness usually occurs in the same muscle groups affected by pain or swelling, with the proximal legs most frequently involved.
- Swelling – Muscle swelling affects 8 to 52 percent of patients with rhabdomyolysis. When it occurs, detectable swelling in the extremities generally develops with fluid repletion. Swelling is less common on hospital admission. Swelling may be due to either,
- Myoedema, which is nonpitting and is apparent at presentation or develops after rehydration
- Peripheral edema, which is pitting and occurs with rehydration (particularly in patients with Acute Kidney Injury or AKI)
- Limb induration is occasionally present.
- Urine — Dark-colored urine (red to brown, “tea-colored,” “cola-colored”) is one of the classic signs of rhabdomyolysis (see ‘Classic triad’above), but it occurs in ≤10 percent of cases. Urinalysis is required to distinguish myoglobinuria (from rhabdomyolysis) from hematuria. (See “Urinalysis in the diagnosis of kidney disease” and ‘Urine findings and myoglobinuria’ below.)
- Myoglobin, a heme-containing respiratory protein, is released from damaged muscle in parallel with CK. Myoglobin is a monomer that is not significantly protein bound and is therefore rapidly excreted in the urine, often resulting in the production of red to brown urine. It appears in the urine when the plasma concentration exceeds 1.5 mg/dL. Visible changes in the urine only occur once urine levels exceed from approximately 100 to 300 mg/dL, although it can be detected by the urine (orthotolidine) dipstick at concentrations of only 0.5 to 1 mg/dL.
Enzymes contained in muscle tissue are released that can be measured in a blood sample helping to confirm the diagnosis. Creatine Kinase (CK) is often elevated to extreme levels and can be used to follow the evolution of the process. Creatine phosphokinase (CPK) is also used to identify damage to skeletal muscle:
Summary – CK vs. CPK Blood Test
Creatine kinase (CK) and creatine phosphokinase (CPK) are two enzymes in the body. They are mainly found in the skeletal muscles, heart, and brain. They catalyze the phosphorylation of creatine. CK blood test detects the presence of creatine kinase, while CPK blood test detects the presence of creatine phosphokinase in the bloodstream. Creatine kinase blood test measures the CK-MM, CK-MB, and CK-BB, and the presence of these enzymes detects health problems or damage in skeletal muscles, heart, and brain tissue, respectively. CPK test measures CPK 1, CPK 2, and CPK 3, and the presence of these enzymes detects any damage or problem in the brain, heart, and skeletal muscles, respectively. So, this summarizes the difference between CK and CPK blood test. https://www.differencebetween.com/what-is-the-difference-between-ck-and-cpk-blood-test/
Myoglobin is released from the degenerating muscle and travels to the kidneys where it has a toxic effect on the renal tubules resulting in renal failure in some patients. (“Myoglobin is a protein that’s found in your striated muscles, which includes skeletal muscles (the muscles attached to your bones and tendons) and heart muscles. Its main function is to supply oxygen to the cells in your muscles (myocytes).” https://my.clevelandclinic.org/health/diagnostics/22142-myoglobin) Urine myoglobin can be detected using a bedside test to detect cell free myoglobin as opposed to hemoglobin which is accompanied by red blood cells. White blood cell counts can be markedly elevated.
Management of moderate and severe cases can be very challenging as the physiology is very disturbed with elevated potassium blood levels, metabolic acidosis, and renal failure.
Dr. Singh’s graphic identifies multiple causes of rhabdomyolysis which fit into two broad categories, those involving physical injury or those related to other, less well-understood mechanisms.
Traumatic causes involve loss of blood flow and subsequent precipitously lowered muscle tissue oxygenation as a result of dangerously elevated tissue pressure (compartment syndrome) or disruption of arterial blood flow in major blood vessel(s) which could be called Macroangiopathy (pathology involving large arteries).
The COVID-19 Spike-Producing Gene Therapy (SPGT) products belong to the latter group of associated and probably causative agents. Interestingly, the Kamura, et al. paper below attributes rhabdomyolysis to pathology occurring in small blood vessels, Microangiopathy, caused by mRNA1273.
This article will examine the evidence supporting this assertion with a review of published case reports and the Vaccine Adverse Event Reporting System (VAERS).
II. Literature Clinical Pathological Case (CPC) Reports
Literature Case Report 1: Myositis at the Injection Site of COVID-19 “Vaccine”
Theodorou, et al. reported a case of muscle inflammation at the injection site the second dose of “COVID-19 vaccine” in a 56-year-old woman.

Theodorou DJ, Theodorou SJ, Axiotis A, Gianniki M, Tsifetaki N. COVID-19 vaccine-related myositis. QJM. 2021 Oct 7;114(6):424-425. doi: 10.1093/qjmed/hcab043. PMID: 33647971; PMCID: PMC7989152.
A 56-year-old, non-diabetic woman with no evidence of prior SARS-CoV-2 infection presented with profound left upper arm pain, soreness and curtailed movement. Because of disabling pain, she could hardly carry her handbag. The patient reported no unaccustomed or vigorous exercise or heavy manual labor prior to the onset of symptoms. Pain had developed 8 days after a second dose of COVID-19 vaccine into her deltoid muscle and produced decreased range of motion and progressive weakness.
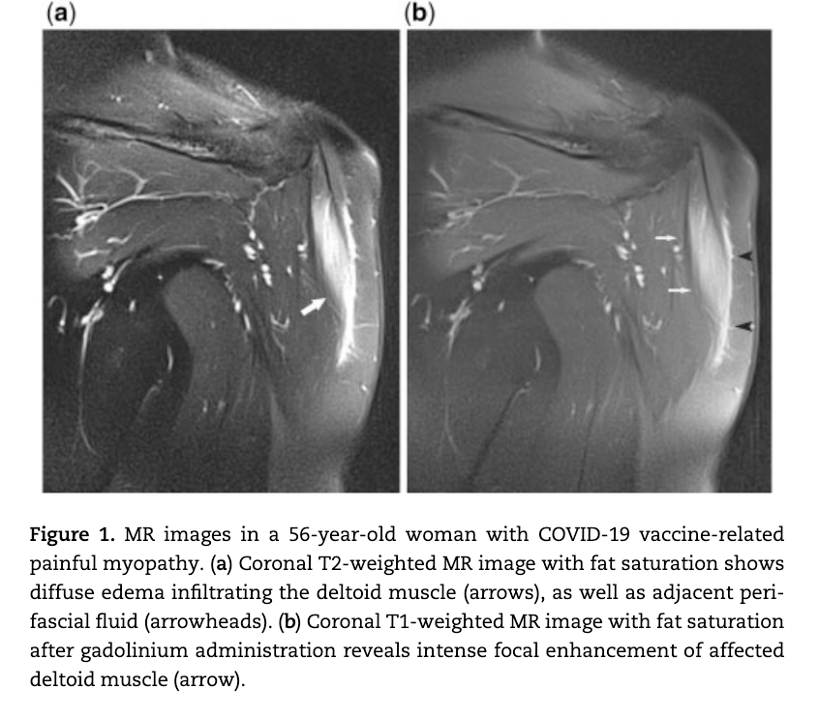
Case 1 Analysis
MRI study following COVID-19 “vaccine” showing edema formation in an inflammatory muscle reaction, myositis, without the lysis or breakdown of the deltoid muscle. No biopsy was obtained in this case so the pathomechanism, autoimmunity versus inflammation versus vasculitis, was not determined. The term myositis implies an inflammatory process possibly associated with lymphocytic infiltration, another of the pathologic processes identified in the Burkhardt series. (War Room/DailyClout Pfizer Reports #56 and #58)
This case gives some insight into the muscular soreness that is commonly reported in the clinical trials under the catchall term of “reactogenicity,” as in this histogram (Below) from the Phase 2/3 trial reported by Pollack, et al. (https://www.nejm.org/doi/full/10.1056/NEJMoa2034577) Certainly myositis and the more severe rhabdomyolysis can cause “Muscle Pain.” A creatine kinase level would have been interesting in these cases and might have been positive in some or many cases. Did use of the concept of “reactogenicity” prevent discovery of myositis and rhabdomyolysis?
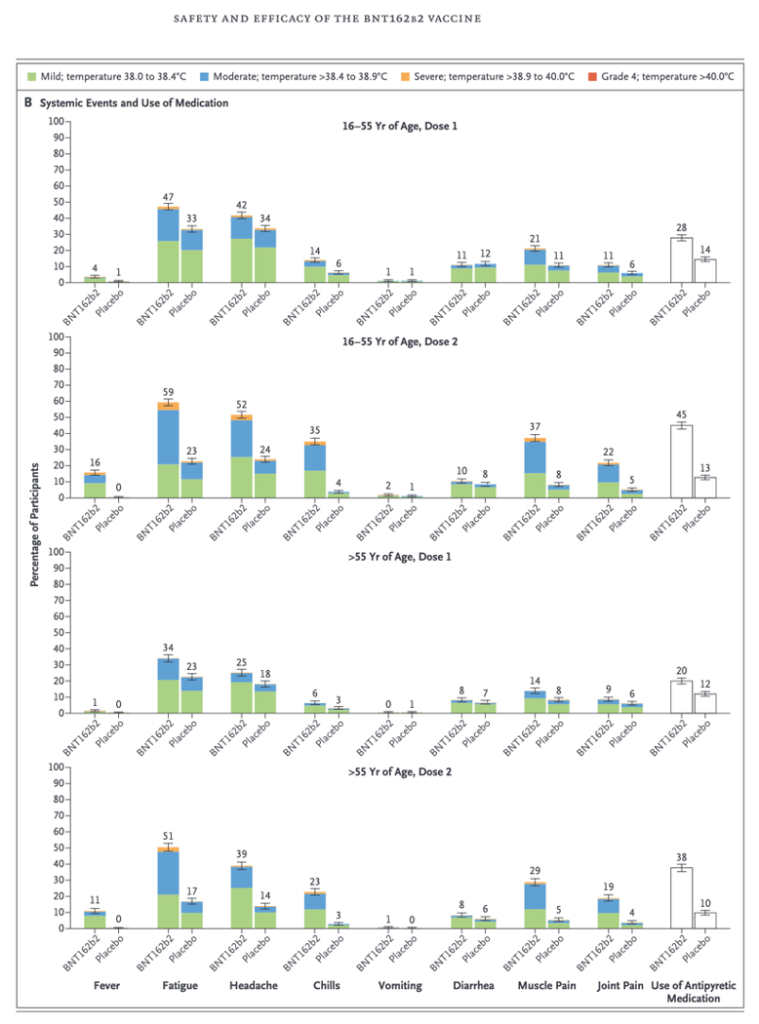
Data from the Pfizer Phase 2/3 clinical trial (Pollack, et al.) show muscle pain occurs in 14-37% of subjects in the clinical trial that led to Food and Drug Administration (FDA) authorization for widespread use under an Emergency Use Authorization (EUA) in December of 2020.
Case Report 2: Myocarditis, Pulmonary Hemorrhage, Myositis, and Rhabdomyolysis.
Al-Rasbi, et al. report a severe but non-fatal case of multiple organ involvement following a single dose of BNT162b2 (Pfizer) in a 37-year-old man
A 37-year-old man presented to the Emergency Department (ED) with a three-day history of back pain and a one-day history of left upper limb swelling with paresthesia and shortness of breath, 12 days after receiving the first dose of Pfizer/BioNTech BNT162b2 mRNA COVID-19 vaccine.
He was diagnosed with severe myositis complicated with rhabdomyolysis and non-oliguric acute kidney injury, thrombocytopenia, myocarditis with pulmonary edema, and pulmonary hemorrhage.
Screens for potential toxic, infectious, paraneoplastic, and autoimmune disorders were unremarkable. The patient was treated with a five-day course of intravenous methylprednisolone and intravenous immunoglobulin, with a good response.
He was hospitalized for 16 days and discharged home on a tapering dose of oral prednisolone for six weeks.
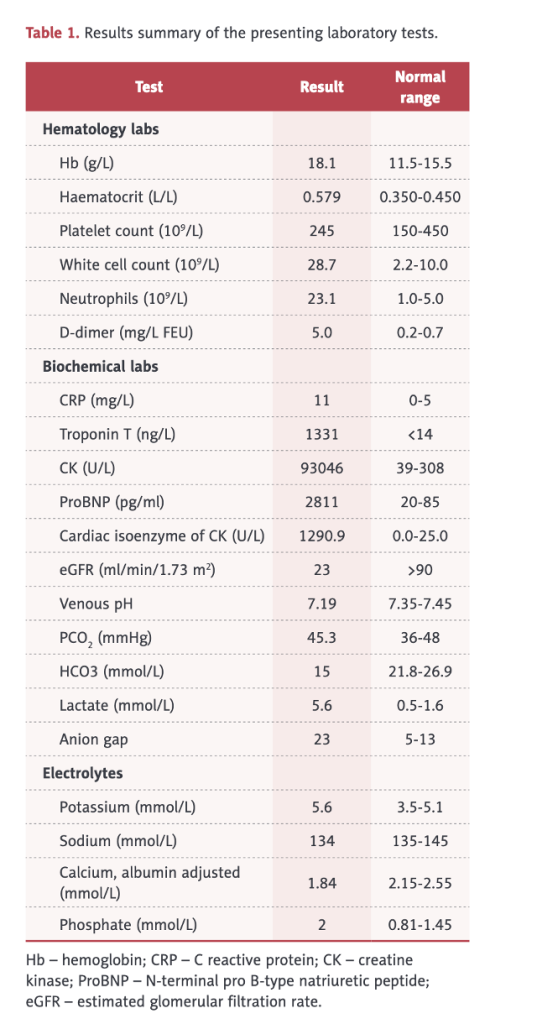
Table 1 (below) shows an elevated white blood cell (WBC) count of 28,700 (2.2-10.0), elevated neutrophils, elevated hemoglobin and hematocrit, d-dimer, C-Reactive Protein (CRP), CK of 93,046, elevated troponin, cardiac isozymes, reduced kidney function, and acidosis.
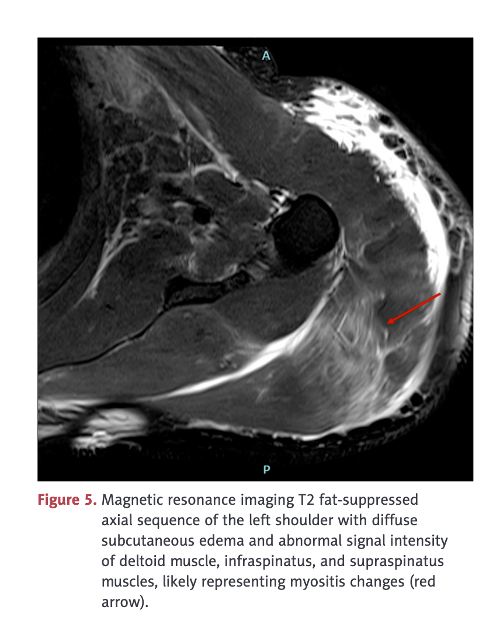
His left, upper arm at the vaccination site was markedly swollen, stiff, warm, and tender, with mild skin erythema and restricted movement at the left shoulder and elbow joints. The left upper arm circumference was 40 cm, and the right upper arm circumference was 33 cm.
Pulmonary vasculitis was diagnosed on bronchoscopy.
Case 2 Analysis
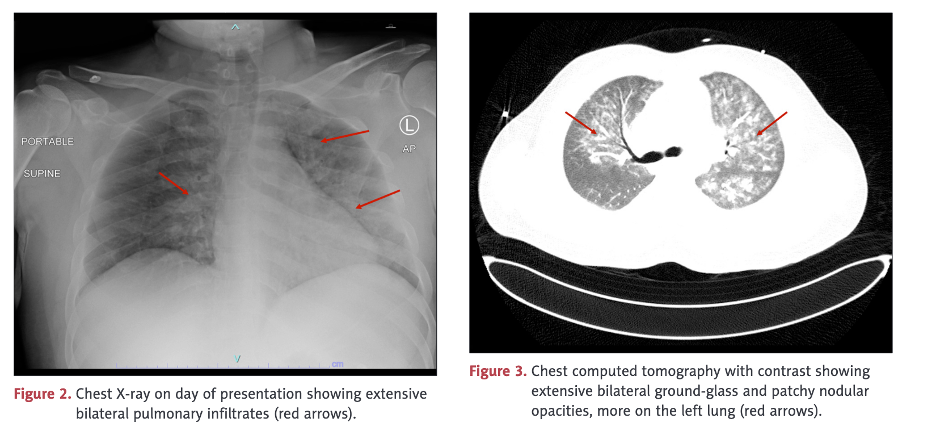
This case illustrates involvement of multiple organs/systems including skeletal muscle, heart, lungs, kidneys, and clotting. In this case, there was not only rhabdomyolysis but also pulmonary hemorrhage and myocarditis with abnormal electrocardiogram, reduced ejection fraction, pulmonary edema on chest X-ray, elevated troponin, and left ventricular hypertrophy.
His Creatine Kinase (CK) was almost 100,000 indicating severe muscle breakdown. His white cell count was 28,700 with 23,100 (80%) neutrophils which, along with a CRP of 11, indicated a severe inflammatory process.
Miraculously, he survived and was discharged after 16 days. There was close temporal and physical proximity of myositis evolving into rhabdomyolysis with a single Pfizer BNT162b2 injection.
A follow-up examination might reveal substantial residuals including reduced cardiac function, loss of shoulder motion and muscular atrophy, and reduced pulmonary capacity.
Literature Case Report 3: Thrombotic Microangiopathy, Rhabdomyolysis, Hepatic Infarction, Gastrointestinal Hemorrhage, Myoglobinuric Renal Failure, Possible Autoimmune Glomerulonephritis

Kamura, et al. report the case of a 57-year-old, otherwise healthy male who presented with subacute thigh pain two weeks after a first dose of Moderna’s mRNA1273 (COVID “vaccine”). On physical examination, he had bruising of both thighs. An MRI showed marked fluid accumulation in the large thigh muscle group called the quadriceps.
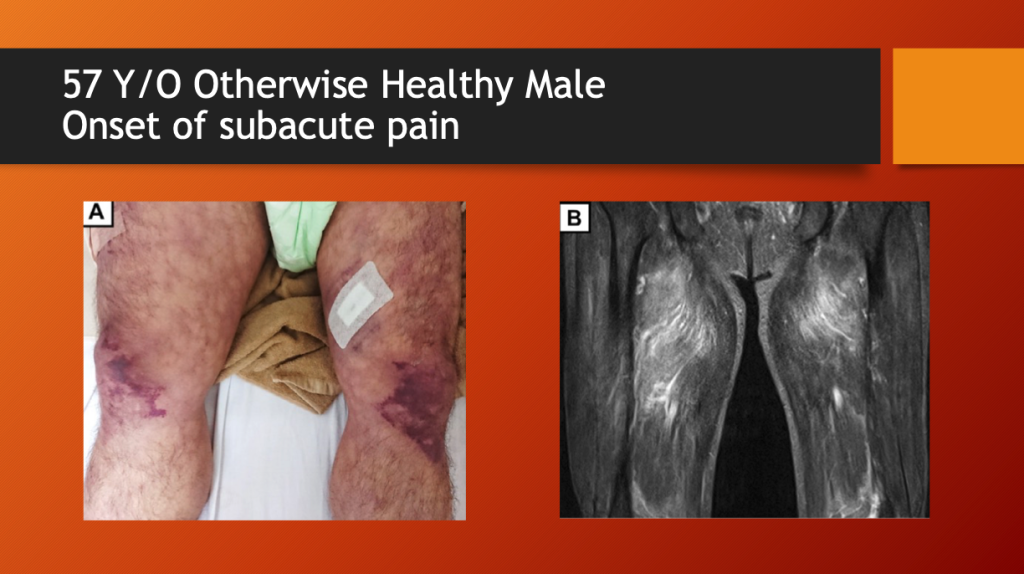
Figure 1. (A) Livedo reticularis on admission. [Livedo reticularis refers to various conditions in which there is mottled discolouration of the skin. It is described as being reticular (net-like, lace-like), as cyanotic discolouration surrounds pale central skin. https://dermnetnz.org/topics/livedo-reticularis] (B) Magnetic resonance imaging findings of the extremities on T2 weighted-imaging demonstrates increased signal intensity in the thigh muscles, consistent with myositis. Note the markedly positive fluid signal in both quadriceps muscles consistent with rhabdomyolysis.
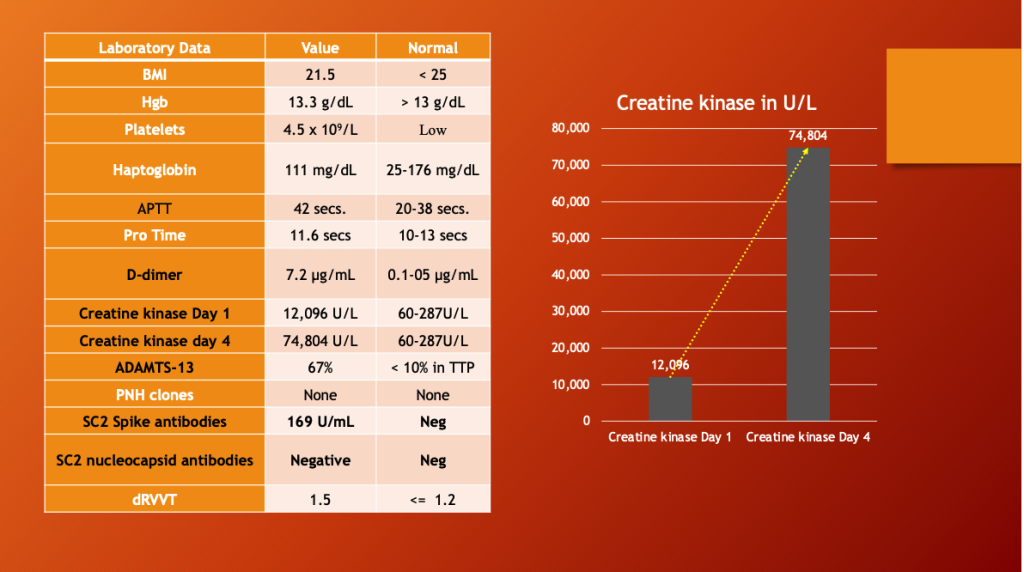
The patient had low platelets, elevated d-dimer, Activated Partial Thromboplastin Time (APTT), and markedly elevated muscle enzyme Creatine Kinase, which rose more than six times higher over the next four days, right side. COVID-19 was ruled out with a negative anti-nucleocapsid antibody test, and mRNA was implicated by the SARS-CoV-2 Spike antibody result.
Additional studies:
- Antibodies related to immune myositis and antiphospholipid syndrome (APS)were negative.
- Bone marrow biopsy revealed normal marrow cellularity.
- Computed tomography (CT) of the abdomen revealed massive ascites that was negative for malignant cell invasion.
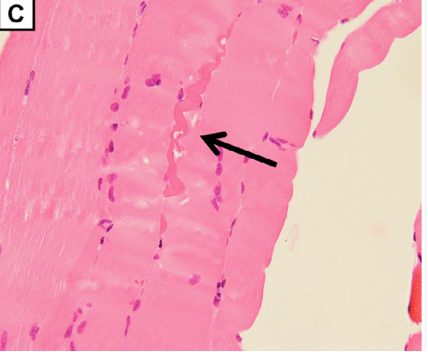
(C) Quadriceps femoris biopsy showed slight rhabdomyolysis without inflammatory cell infiltration (magnification: × 400).
A biopsy of the quadriceps muscle was unimpressive given the extensive involvement of his thigh muscles, possibly due to sampling error.
His hospital course was complicated by severe renal failure, gastrointestinal bleeding, and ongoing release of toxic breakdown products from these large muscle groups into the systemic circulation.
Case evolution:
- Renal dialysis was begun.
- Renal and hepatic infarction was identified on CT scan.
- Gastrointestinal bleeding.
In spite of efforts to save this man, he died after 18 days in the hospital, 32 days after one dose of mRNA1273.
Autopsy Findings:
Italics added.

(D) Autopsy reveals acute to subacute infarction with scattered white changes surrounded by hemorrhagic areas. Liver cross section.
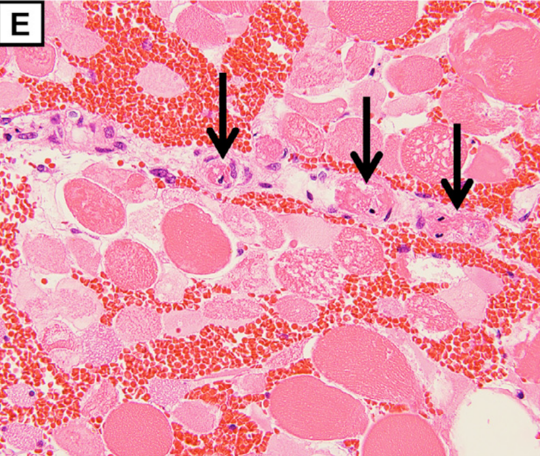
The pathological findings demonstrate degeneration and necrosis of the myocytes (E; magnification: × 200) and hepatocytes (F; magnification: × 100). Both iliopsoas musclesand the right quadriceps femoris were extensively necrotic with massive hemorrhage. Histological examination revealed multiple small arterial thrombosis, degeneration, and necrosis of the myocytes.
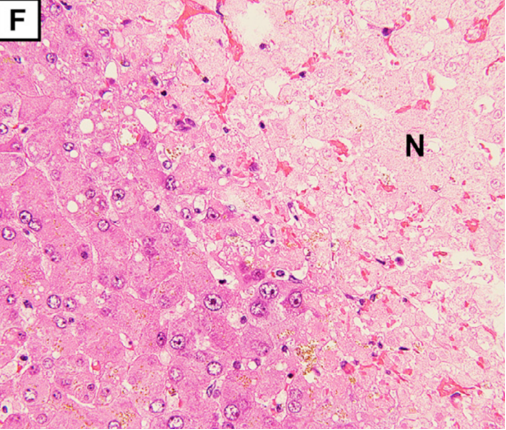
Multiple small arterial thrombosis, degeneration, and necrosis of the hepatocytes (Figure 1F) (F; magnification: × 100).
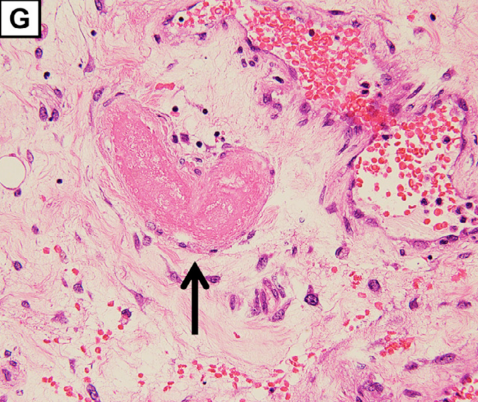
Extensive hemorrhagic necrosis of the mucosa involves the entire intestinal tract, and there is microvascular thrombosis (G; magnification: × 100. The arrow points to the thrombosis in the duodenum).
Microangiopathy, thrombosis in this case, was found in skeletal muscle, liver, and the intestinal tract and points to an underlying pathologic process involving small arteries. The Burkhardt Group (War Room/DailyClout Pfizer Reports #56 & #58) identified blood vessels as a target for Spike proteins transcribed from genetic instructions contained in SPGT products. Blood vessels are known to contain the ACE2 receptor, angiotensin converting enzyme II, that serves as the docking site for Spike proteins to gain entry to host cell machinery.
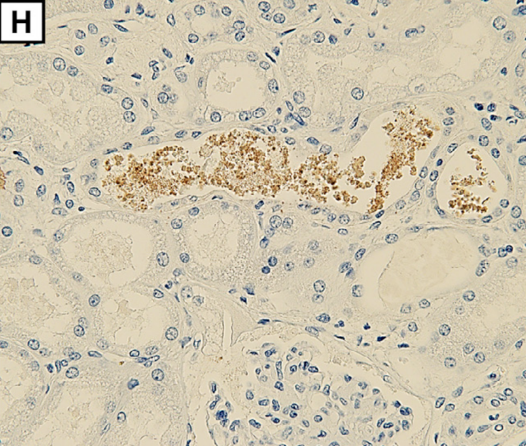
Myoglobin deposition is observed in renal tubules (H; magnification: × 400).
Myoglobin is released from decaying muscle tissues, enters the circulation, and collects in kidneys where it has a toxic effect leading to renal failure requiring hemodialysis. Myoglobinuria, myoglobin in the urine, and renal compromise are secondary effects of the rhabdomyolysis rather than a result of SPGT products directly.
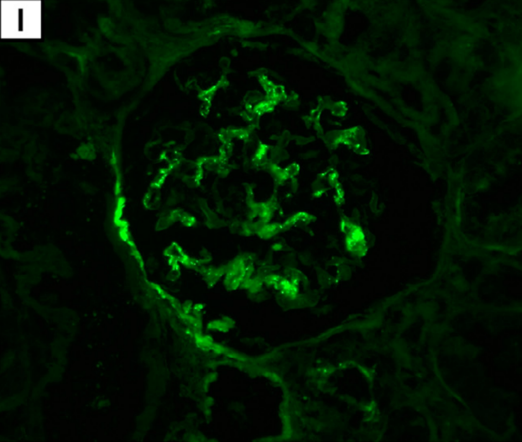
Immunofluorescence reveals C3 deposits in renal glomeruli (I; magnification: × 400).
Complement C3 deposits in renal glomeruli are suggestive of autoimmunity from the mRNA1273 injection giving two mechanisms of renal damage, primary autoimmunity in the form of C3 nephropathy and secondary myoglobin nephropathy. (Caravaca-Fontán F, Lucientes L, Cavero T, Praga M: Update on C3 Glomerulopathy: A Complement-Mediated Disease. Nephron 2020;144:272-280. doi: 10.1159/000507254, https://www.karger.com/Article/FullText/507254)
The final diagnosis was “vaccine-induced TMA with rhabdomyolysis as an initial symptom” based on:
“…extensive hemorrhagic necrosis of the mucosa that involved the whole intestinal tract was thought to be the consequence of thrombosis of involved arterioles (Figure 1G, arrowhead). Microscopic findings were consistent with the features of a thrombotic microangiopathy (TMA).”
Case 3 Analysis:
This case is important because it temporally links a devastating and fatal case of rhabdomyolysis to a single dose of mRNA1273. Two or more LNP/mRNA-linked pathologic processes, angiopathy and autoimmunity, possibly involved four organs, skeletal muscle, liver, intestine, and kidneys. We know from the Burkhardt Group’s histopathology work that vascular injury and autoimmunity are closely linked to Spike producing drug treatments. (https://dailyclout.io/report-56-autopsies-reveal-the-medical-atrocities-of-genetic-therapies-being-used-against-a-respiratory-virus/ and https://dailyclout.io/report-58-part-2-autopsies-reveal-medical-atrocities-of-genetic-therapies-being-used-against-a-respiratory-virus/)
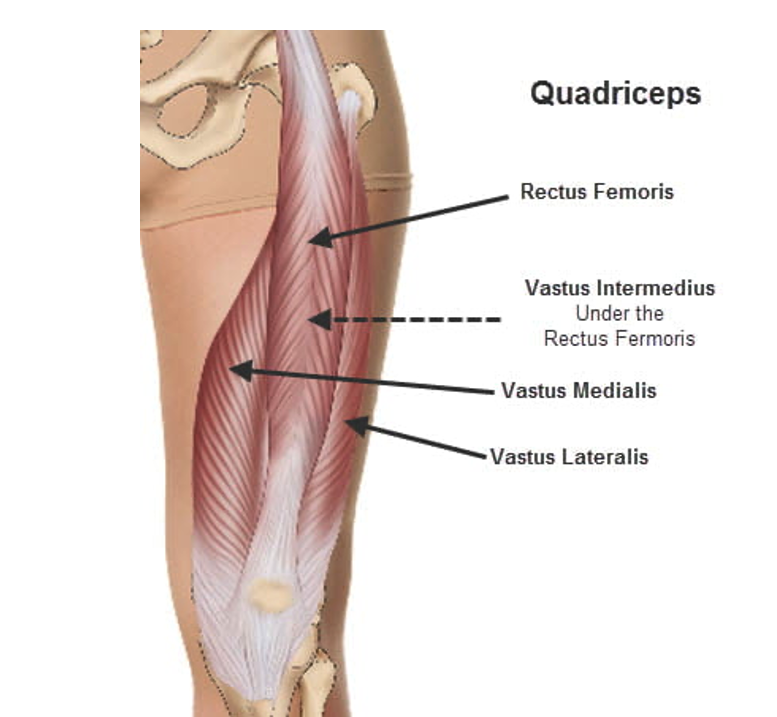
The quadriceps are comprised of four separate muscles, rectus femoris, vastus intermedius, vastus lateralis, and vastus medialis, and, as such, constitute the first or second largest muscle groups in the human body. This case also involved portions of two other muscles, the psoas and iliacus muscles. Considering bilaterality, there were potentially 10 individual muscles involved in the rhabdomyolysis although precise muscle identification was not discussed.
The involvement of these large muscle groups, along with the hemorrhagic process in the gastrointestinal tract, made this a fatal process no matter what treatments were instituted.
A 57-year-old reportedly otherwise healthy man died from vascular and possibly autoimmune processes caused by mRNA gene therapy.
III. What Did the Rats Reveal in Pfizer Pre-Clinical Study 31866?
Were the adverse effects of BNT162b2 on muscle tissue identified in the Pre-Clinical Studies? Pfizer study 38166 answers affirmatively.
The Pfizer documents contain results from a 17-day study of repeat dose injections of BNT162b2 in Wistar Han rats. Myonecrosis and inflammation were identified histopathologically. The appearance was described as “Jellied” (Table 3), which is what rhabdomyolysis might look like after 17 days.
Pfizer Document 31866:
Wistar Han Rat Histopathology Findings
REPEAT-DOSE TOXICITY STUDY OF THREE LNP-FORMULATED
RNA PLATFORMS ENCODING FOR VIRAL PROTEINS
BY REPEATED INTRAMUSCULAR ADMINISTRATION
TO WISTAR HAN RATS
Gross Examination
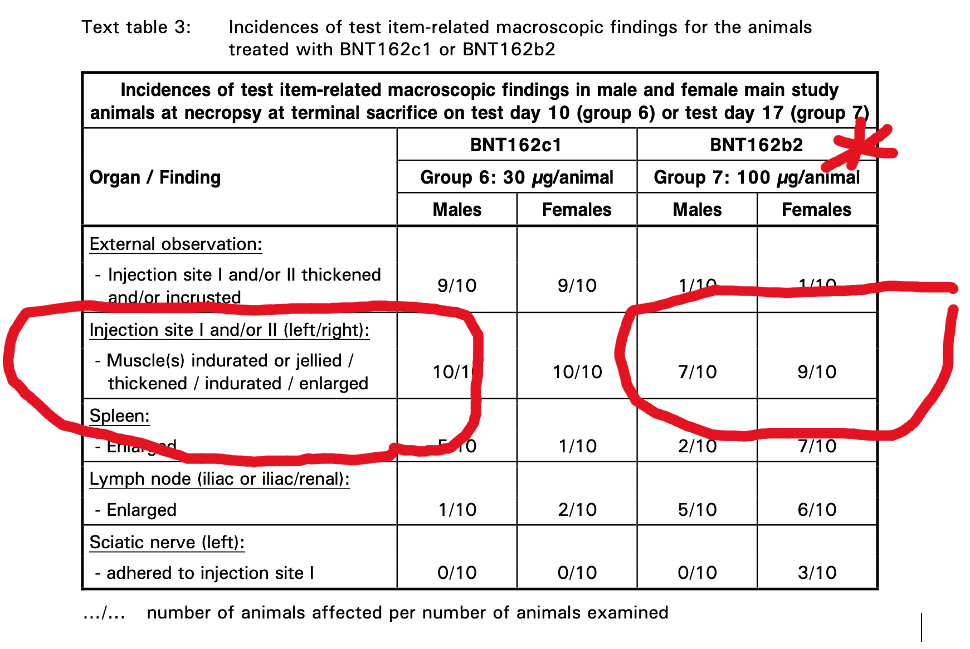
Question: Is “Jellied Muscle” the same as myositis or rhabdomyolysis?
Answer: Probably.
“Jellied” is probably a good physical description of muscle that is lysing (disintegrating) on inspection of the gross specimen examined with the naked eye. The microscopic histopathology is also confirmatory for muscle damage caused by BNT162b2.
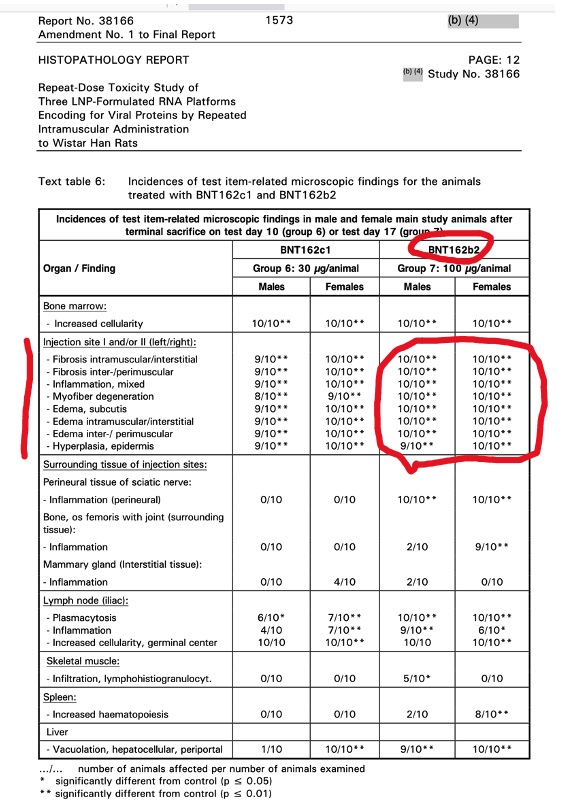
p. 1567
Table 6 above gives the histopathological findings with Pfizer’s BNT162b2, the drug administered to billions of humans: Fibrosis, Inflammation, Myofiber Degeneration were present at the injection site.
Pfizer Report 31866 describes additional physiological changes including abnormalities in blood and the clotting mechanism.
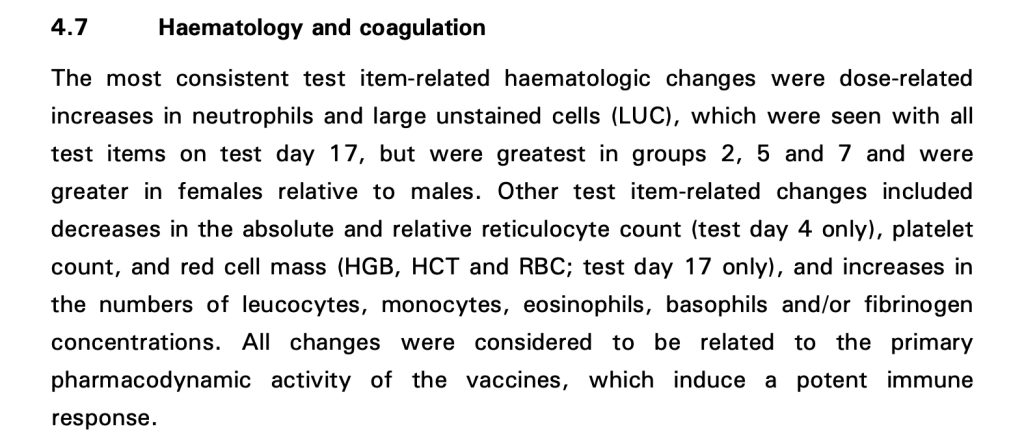
An interesting conclusion is contained in the last sentence: “All changes were considered to be related to the primary pharmacodynamic activity of the vaccines, which induce a potent immune response.”
How was this data presented at the December 10, 2020, Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting regarding the Emergency Use Authorization for BNT162b2?
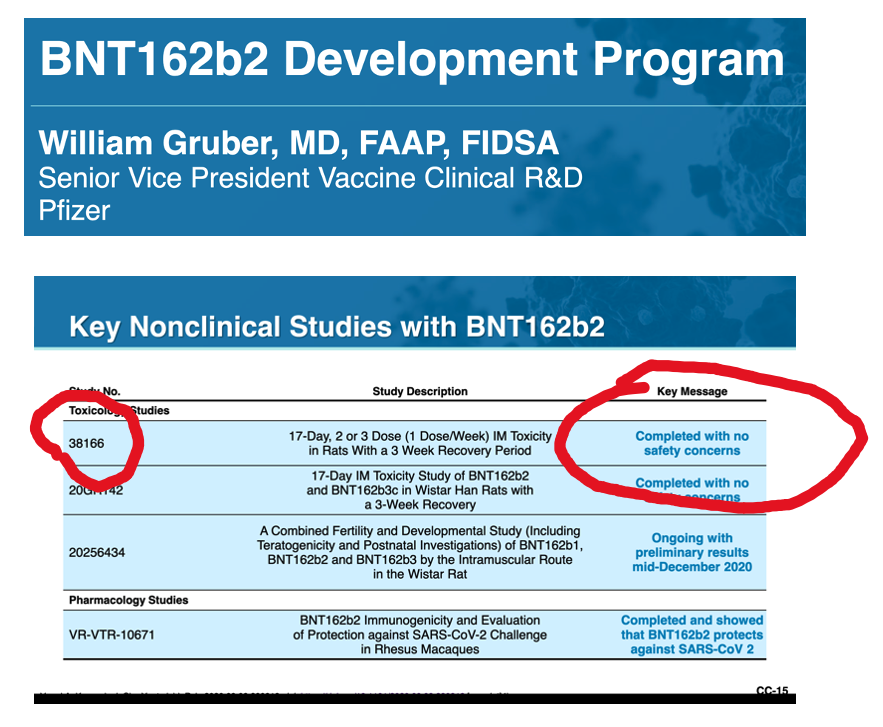
Answer: Key Message, “Completed with noconcerns.”
IV. Rhabdomyolysis in Vaccine Adverse Event Reporting System (VAERS)
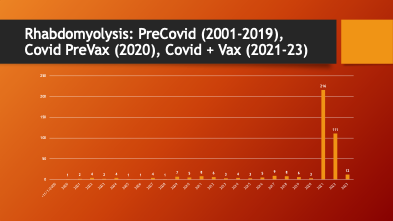
VAERS was accessed to see if there was a change in the pattern of reporting for rhabdomyolysis. Data were available from 2001 through March of 2023.
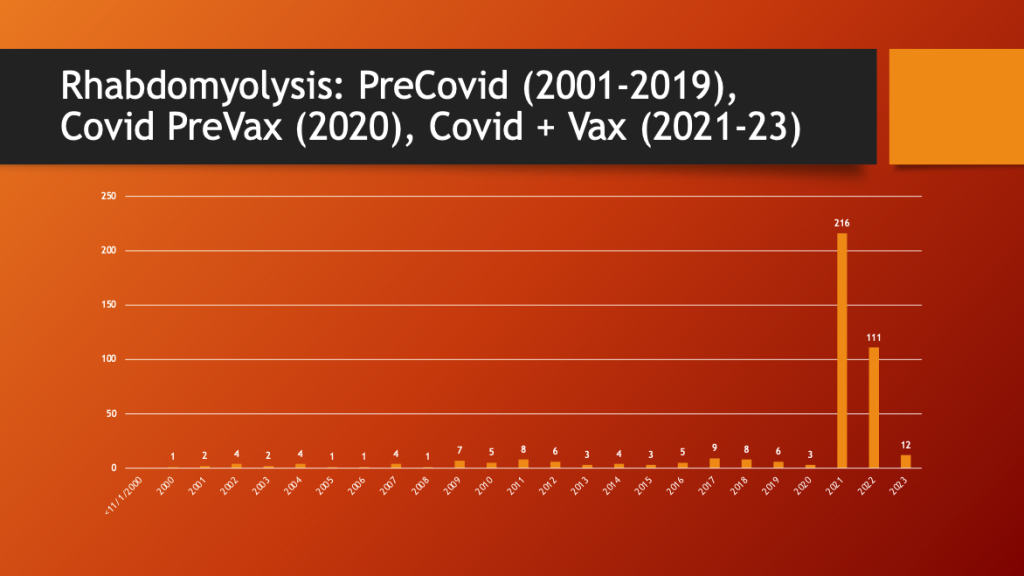
Maria Ziminsky assisted with the access and analysis of the VAERS data.
79% of all reported rhabdomyolysis cases occurred in the two complete years (2021 and 2022) after the EUA was approved in December of 2020.
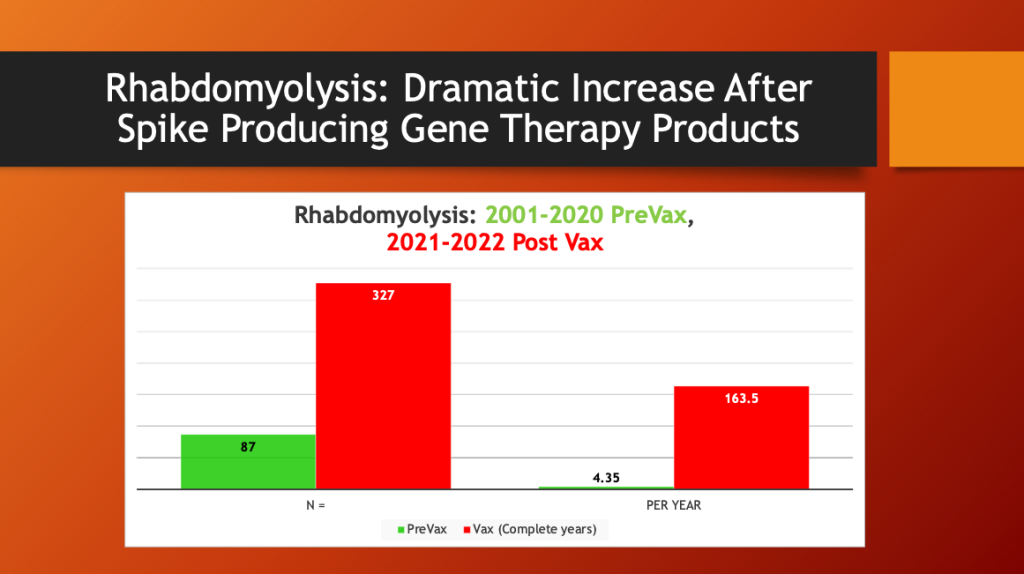
Maria Ziminsky assisted with the access and analysis of the VAERS data.
A dramatic, 37-fold increase in the annual rate of cases of rhabdomyolysis occurred after mass inoculation with Spike Producing Genetic Therapy Products began in December 2020. COVID-19 (2020) did not cause an increase in rhabdomyolysis reporting in VAERS compared with years 2001-2020.
Next, we examine a sample of cases from VAERS.
V. VAERS Clinical Pathological Case (CPC) Reports
VAERS Case Report #1: (Fatality) Rhabdomyolysis Twice after each mRNA1273 Injection

This is the case of a 59-year-old woman with the following chronology:
- 4/2/2021 – First Dose of Moderna mRNA injected.
- Hospitalization 4/30/21 to 5/19/2021 for rhabdomyolysis.
- 9/20/2021 – Second Dose of Moderna mRNA injected.
- 10/8/2021 – Hospitalized with rhabdomyolysis.
- Acute renal failure, hemodynamic instability.
- Death – 10/14/2021.

This record documents the fatal outcome following the second administration of mRNA1273, a drug that caused rhabdomyolysis after a prior administration. Her VAERS record was flagged for “Inappropriate Product Administration,” but the circumstances surrounding the second dose were not given.
Did this unfortunate woman have a second dose so she could continue to earn a livelihood, take a cruise, visit an elderly relative? We do not know.
VAERS Case Report #2: Ipsilateral Rhabdomyolysis and BNT162b2 Injection Site
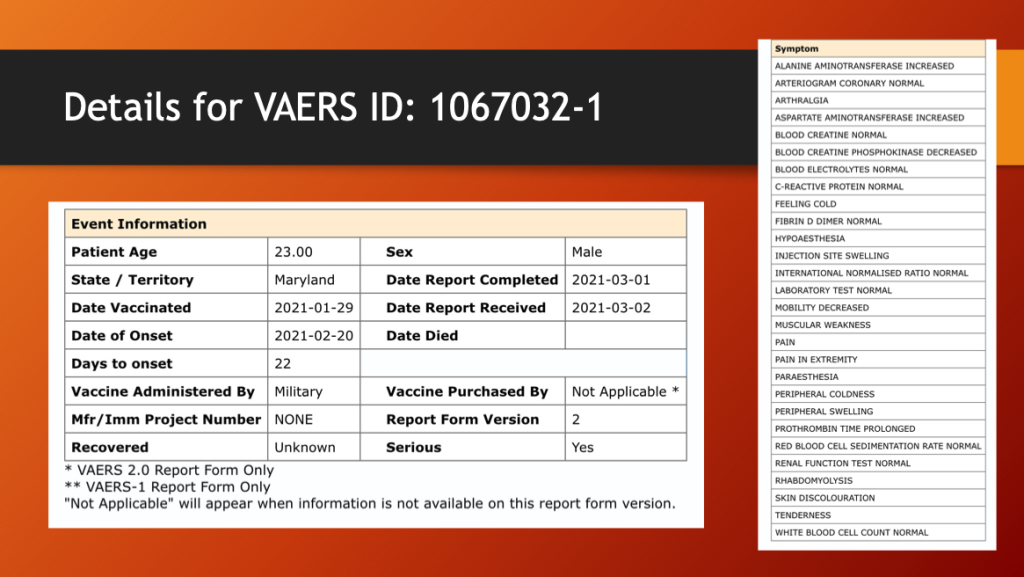
This case concerns a 23-year-old male who received Pfizer BNT162b2 administered by the United States Military, presumably because he was ordered to as a member of the Armed Forces.

This man received a second dose of BNT162b2 in his left arm on January 29, 2021, 18 days after a first dose. On February 20, 2021, he was seen for two days of worsening numbness, coldness, discoloration of his left hand, and moderate pain.
The next day he was admitted for rhabdomyolysis manifest by moderate pain, discoloration, swelling, and motor loss in his left upper extremity. His CK level was elevated at 31,924. Cardiovascular contrast study (CTA) was interpreted as normal. He received three liters of intravenous fluids and was discharged with a CK of 14,653 (very high). What was this soldier’s renal function? What impairment and disability resulted from this Adverse Event?
VAERS Case Report # 3: Rhabdomyolysis in a Child

Case #3 involved a 12-year-old male and was included to document rhabdomyolysis in a pre-teen. After he received his second dose of an unknown COVID-19 vaccine approximately 15 days previously, he was hospitalized briefly as his CK dropped to “acceptable” levels.
There are hundreds of similar cases beginning in 2021.
V. Discussion
There is strong evidence causally linking COVID-19 vaccines to both inflammation in muscles and more a damaging condition known as rhabdomyolysis from published case reports and the government-maintained Vaccine Adverse Events Reporting System (VAERS). Rhabdomyolysis was rarely reported prior to 2021 when five or six cases would have been above average.
COVID-19 arrived early in 2020 yet the number of cases of rhabdomyolysis did not rise above the range in the two previous decades.
Widespread use of Spike-Producing Gene Therapy products began at the end of December of 2020, giving a full calendar year of dosing in 2021.
What happened after mass inoculation began was a huge jump in the number of cases of rhabdomyolysis reported to the VAERS database. For two decades, the annual number of rhabdomyolysis cases averaged 4.35 per year. After the widespread COVID-19 inoculation program began in late December 2020, this rate jumped to 163.5 per year.
Early signs of adverse effects on muscle tissue were present in Pfizer’s Pre-Clinical Study 31866. In the clinical trials, complaints of muscle pain were common and were attributable to “reactogenicity,” a curious aberration in the vaccine world as how does one know if muscle pain at the injection site or remote to the injection site is a significant medical problem such as myositis or rhabdomyolysis?
The publicly accessible VAERS database provides case report documentation of what could fall under the “reactogenicity” cluster of signs and symptoms when, in fact, something much more serious was the cause of those findings.
The consequences of rhabdomyolysis can be profound, ranging from permanent impairment and disability to death in about 8% of cases. Case reports document a close temporal connection between the mRNA injections and the onset of rhabdomyolysis days to weeks later. The location of the muscle undergoing degradation is both at the injection site and remote to it.
VAERS Case Report #1 documented a fatal sequence of a first course of rhabdomyolysis after mRNA followed by a second dose which proved to be fatal.
Rhabdomyolysis following COVID-19 gene therapy products comes as an isolated adverse event (AE) or in concert with other adverse events. Associated AEs have different pathologic processes — such as destructive inflammation, autoimmunity, coagulopathy, and vasculitis in other tissues and organs. Multiorgan failure is a feature of moderate and severe cases of rhabdomyolysis, thus presenting a medical management challenge.
Once rhabdomyolysis initiates, there is little to do to slow or stop the process. Medical care consists of managing fluid, electrolytes, and a host of metabolic and chemical problems. Dialysis is necessary in some cases, but the extent of the muscle degradation can be overwhelming. There are no predictive screening tests for rhabdomyolysis.
Actual measurement of the incidence of drug -related rhabdomyolysis is not provided by either case reports or VAERS. VAERS numbers underestimate the true rate of occurrence of this dangerous condition, but most estimate a factor of 10 or more giving an estimated incidence of 3,270 cases in the two full years of mass COVID-19 gene therapy inoculations with an estimated 261 fatalities ( 8%).
How many deaths is too many?
What is the morbidity in the survivors? These are vital questions that need answering.


No comments:
Post a Comment