Report 72: “Other AESIs” Included MERS, Multiple Organ Dysfunction Syndrome (MODS), Herpes Infections, and 96 DEATHS. 15 Patients Were Under Age 12, Including Six Infants.
May 30, 2023 • by Joseph Gehrett, MD; Barbara Gehrett, MD; Chris Flowers, MD; and Loree Britt
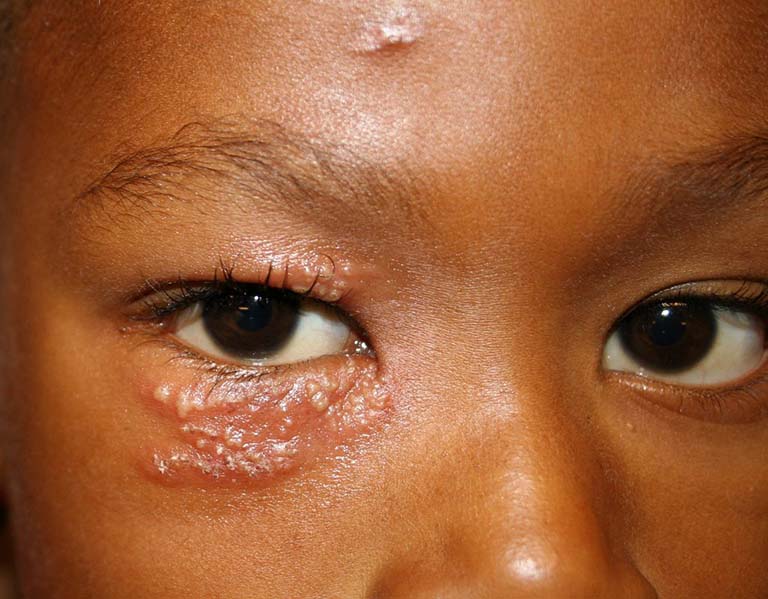
The War Room/DailyClout Pfizer Documents Analysis Project Post-Marketing Group (Team 1) – Joseph Gehrett, MD; Barbara Gehrett, MD; Chris Flowers, MD; and Loree Britt – wrote a report about Other Adverse Events of Special Interest (AESIs) found in Pfizer document 5.3.6 Cumulative Analysis of Post-Authorization Adverse Event Reports of PF-07302048 (BNT162B2) Received Through 28-FEB-2021 (a.k.a., “5.3.6“). This category of AESIs is not related to a specific set of medical conditions or a specific organ. Rather, it contains medical conditions such as herpes virus infections, MERS (Middle East Respiratory Syndrome), MODS (Multiple Organ Dysfunction Syndrome); symptoms such as fever and inflammation; and non-medical-related issues like manufacturing issues.
It is important to note that the AESIs in the 5.3.6 document were reported to Pfizer for only a 90-day period starting on December 1, 2020, the date of the United Kingdom’s public rollout of Pfizer’s COVID-19 experimental mRNA “vaccine” product.
Key points of this report include:
- Death was listed as the “relevant [adverse] event outcome” for 96 individuals in this category.
- Fifteen patients were under 12 years of age, including six infants; and Pfizer’s mRNA “vaccine” was not approved for use in people under age 16 at the time of 5.3.6‘s data collection.
- Of those reports with gender known, 76% were female and 24% were male, a greater than 3:1 female to male ratio.
- There were 391 herpes infectionsreported, including shingles, herpetic eye infections, and non-shingles herpes infections.
- There were 18 Multiple Organ Dysfunction (MODS) adverse events.
- Onset of adverse events, a.k.a. latency, was from within 24 hours to 61 days with half occurring within one day.
- This category included 8,152 patients/cases, which is 19.4% of the total cases reported to Pfizer during its 90-day post-marketing safety surveillance.
- Non-elderly adults had almost six times the number of adverse events seen in elderly adults.
- Fever was the most common adverse event.
- Pfizer concluded: “This cumulative case review does not raise new safety issues. Surveillance will continue.” To date, no follow-up, updated, and comprehensive safety report has been publicly released.
Please read this important two-page report below.
p1 Post Marketing Team Other AESIs MicroReport
https://dailyclout.io/wp-content/uploads/p1-Post-Marketing-Team-Other-AESIs-MicroReport.pdf
p2 Post Marketing Other AESIs MicroReport
https://dailyclout.io/wp-content/uploads/p2-Post-Marketing-Other-AESIs-MicroReport.pdf


No comments:
Post a Comment