EU safety report on Pfizer-BioNTech mRNA vaccine reveals damning data
Sonia Elijah Feb 20 44 9

Republished from Children’s Health Defense Europe.
This article has been revised as of February 22.
The first ever 286-page EU Periodic Safety Update Report (PSUR #1), covering the 6 month period from 19 December 2020 through 18 June 2021 reveals damning safety signals for the Pfizer-BioNTech Covid-19 vaccine (COMIRNATY). It was released via FOIA request from an anonymous reader and provided to the Austrian science and political blog, TKP.
According to the European Medicines Agency definition: ‘PSURs are pharmacovigilance documents intended to provide an evaluation of the risk-benefit balance of a medicinal product at defined time points after its authorisation. The objective of the PSUR is to present a comprehensive and critical analysis of the risk-benefit balance of the product, taking into account new or emerging safety information in the context of cumulative information on risk and benefits.’
It is the MAH (Marketing Authorisation Holder) in this case, BioNTech SE, who are legally required to submit PSURs to the EMA, along with an application fee. The EMA then assesses the information found in the report to determine if any new risks are identified or if the risk-benefit balance has changed.
I have extensively gone through voluminous Pfizer-BioNTech vaccine-related documents, this report was one of the most eyebrow-raising, not just in relation to the damning data but its conclusion that the ‘benefit-risk profile of BNT162b2 remains favourable.’
The damning data
The following is an overview of the total number of cases (post-marketing and clinical trial data) of the 6-month reporting period:
The table below (extracted from the document) shows the number of cases broken down by: gender, age, country, case seriousness, outcome and presence of comorbidities.
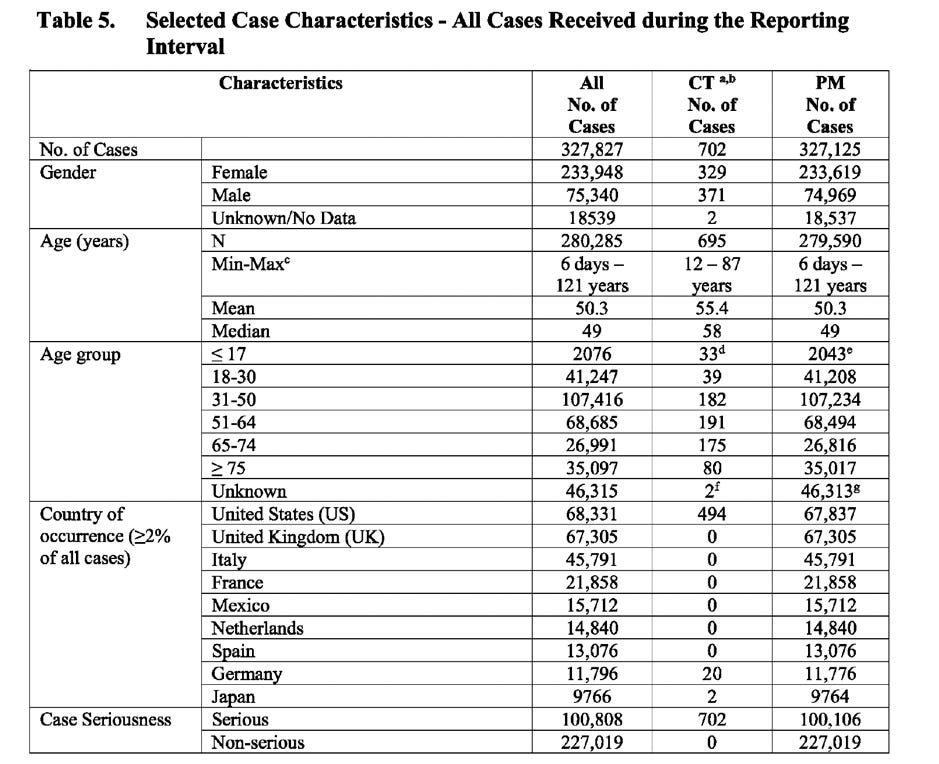
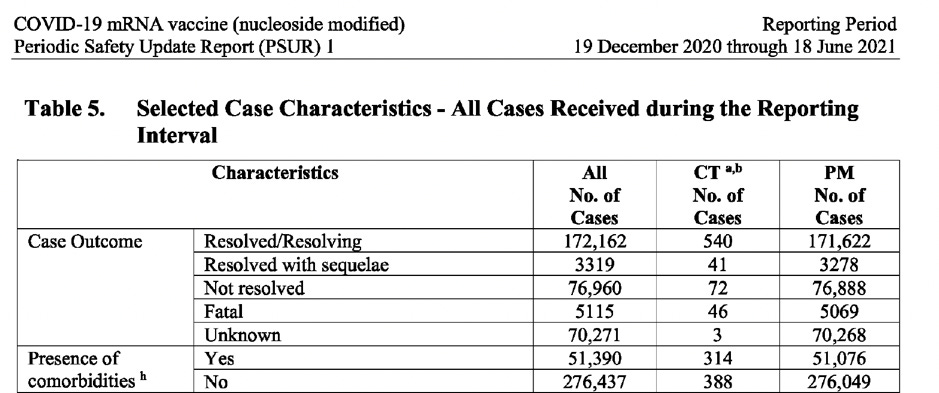
CT: Clinical Trial data PM: Post Marketing data
What’s noteworthy is that similar findings relating to: gender-specific vaccine safety risks for females; individuals (cases) suffering from an average of 3-4 adverse events; high number of reports with either unknown and/or unresolved outcomes; high number of cases with gender unknown and the unusual grouping together of resolved/resolving cases were found when I analysed the Pfizer prepared document for the FDA, entitled, “Cumulative Analysis of Post-Authorization Adverse Event Reports of PF-07302048 (BNT162B2) received through 28 February 2021”.
However, a striking difference between the two reports, is the explosion in the number of cases and events recorded in the EU’s PSUR, which is a staggering seven times higher compared to Pfizer’s submission to the FDA, which covered half the period.
The document states that during the reporting interval, 635,763,682 doses were estimated to have been administered worldwide and adds: “It is not possible to determine with certainty the number of individuals who received BNT162b2 during the period of this review.” Therefore, extrapolating accurate incidence rates is not feasible.
However, an important point to factor in when assessing the data, is the magnitude of underreporting of cases. A Harvard studyconcluded that only 1-13% of serious adverse events are ever reported. Furthermore, a systematic review by Hazell et al., provided ‘evidence of significant and widespread under-reporting of ADRs to spontaneous reporting systems including serious or severe ADRs.’ Therefore, it’s concerning that although the number of cases and events in the reporting interval seem high, the actual numbers may have been far higher.
The affected younger age groups with no comorbidities
It’s a known fact that the majority of people fully recover after contracting Covid-19, particularly those with no comorbidities (no underlying health issues) and who are not elderly. However, the PSUR report reveals an alarmingly different story when it comes to the Pfizer-BioNTech vaccine recipients: a staggering 23% of the cases of those suffering from adverse events after receiving the vaccine, did not recover (they were left unresolved) and for 21% of the cases, the outcome was completely unknown. Also, the highest number of reported cases was in the 31-50 age group, not in the elderly population. When analysing the post-authorization data for fatal cases, broken down by presence/absence of comorbidities, across all age groups up to 64 years, more fatal cases occurred in individuals with no comorbidities. Of those aged 17 years and under, 22 individuals died who had no comorbidities, compared to 1 who did. Only in the 65+ age group do we see the number of fatal cases with comorbidities overtake those without. (See screenshot below).
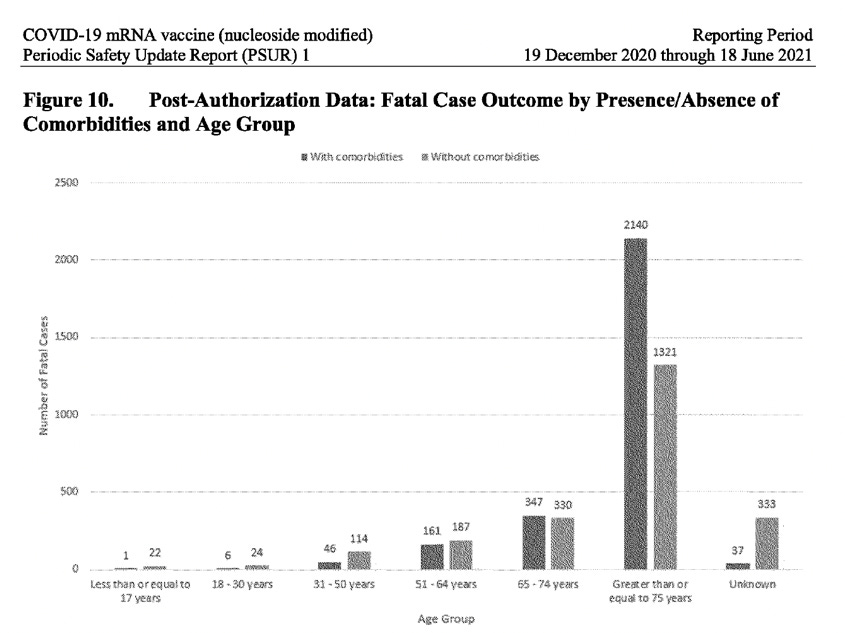
It is highly unusual that the PSUR did not address this obvious safety signal affecting younger age groups but instead glossed over it by saying “overall, the proportion of cases with a fatal outcome is slightly higher when comorbidities are reported.”
The case of Maddie de Garay
When looking at the reporting of the clinical trial data for the 12-15 year age group in the document, an obvious omission has been made, which can be described as the cover up of Maddie de Garay’s case.
In early 2022, I interviewed Maddie de Garay’s mother, Stephanie. Maddie, aged 12 at the time, took part in Pfizer/BioNTech’s clinical trial for 12–15-year-olds. Shortly after taking the second dose, Maddie was left with life-altering injuries, leaving her immobile and dependent on a feeding tube. Maddie’s mother told me first-hand that Pfizer’s principal adolescent trial investigator, Dr Robert Frenck, recorded her daughter’s devastating injuries as “abdominal pain” even though he and the other Cincinnati hospital doctors were aware of all her symptoms.
Notice how ‘abdominal pain’ is one of the PTs (preferred terms) in relevant cases, referenced in the screen shot below, for the clinical trial data for 12-15-year-olds.
The PSUR was signed off by the EU Qualified Person for Pharmacovigilance, Pfizer, Barbara de Bernardi, two months after the reporting cut-off date of 18 June, 2021. It’s unusual how the EMA’s CHMP recommended the Pfizer-BioNTech vaccine for children aged 12-15 years old, a month prior to the finalization of the EU’s first ever Periodic Safety Update Report, on 28 May, with the EMA approving it for this subpopulation just a few days later. Why didn’t they want to wait for this important pharmacovigilance document providing an evaluation of the risk-benefit balance of the vaccine to come out first, before they made their decision?
The bad lots
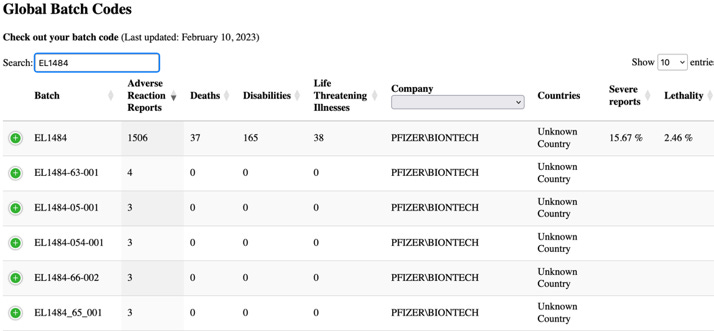
The table above shows the most frequently reported lot numbers in case reports. Notice the frequency of cases reported for certain lot numbers, such as lot number EL1484, which was reported in 16,077 cases. Yet, PSUR goes on to state ‘overall, there were no related quality issues identified during investigations of these lot/batch numbers..and any potential signals indicating a potential relationship between a safety issue and a particular batch lots, and that was not already evaluated as part of other signal activities, would undergo evaluation and escalation as per standard procedures.’
This statement is evasive- even the term “overall” implies there could have been issues related to quality that were identified across different batches. In fact, it was known that they were. I have written extensively on the leaked EMA emails and other Pfizer-BioNTech related documents which revealed that the regulators had concerns about the batch-to-batch variation in vaccine quality right up to the time emergency use authorisation was granted. Significantly higher proportions of truncated mRNA species (not intact) were identified in the commercial lots of the vaccine, compared to those used in the clinical trials. These truncated species were classified as “product-related impurities.”
When searching the database from the website, HowBadIsMyBatch.com, for the referenced lot number above (EL1484)- the following information was found.
The level of severe reports and lethality arising from this lot is shocking, yet the EU document appears to find nothing unusual to report.
Menstrual Disorders
What’s interesting is that the report states “In the final AR [assessment report] of the 5thSMSR [summary monthly safety report] 1 April 2021- 29 April 2021, it was stated that a number of queries have been received about menstrual disorders, especially menorrhagia [prolonged bleeding]. This issue merits further investigation in the upcoming PSUR, which may be a matter of concern for young women..’
It then goes on to state ‘the MAH [BioNTech] is requested to include in this PSUR review a separate ‘post-marketing cases evaluation’ of the cases reporting a menstrual disorder, which should also include a sub-analysis of cases divided between post-menopausal cases and menstrual disorder cases. Causality assessment should be provided per case for a least the serious cases…’
This information was allegedly provided in Appendix 6B.6 ‘MENSTRUAL DISORDERS’
However, the FOIA release of this PSUR did not include any of the important appendix items.
The conclusion to the PSUR investigation of menstrual disorders, is remarkably dismissive and what some would view as even offensive: ‘These are likely to be associated with psychological distress and stress related to the pandemic, weight gain, longer working hours and dietary changes..’
A report published in the British Medical Journal, shortly after this PSUR was approved, stated ‘Changes to periods and unexpected vaginal bleeding are not listed, but primary care clinicians and those working in reproductive health are increasingly approached by people who have experienced these events shortly after vaccination. More than 30 000 reports of these events had been made to MHRA’s yellow card surveillance scheme for adverse drug reactions by 2 September 2021, across all covid-19 vaccines currently offered.’
Furthermore, in July 2022, a systematic review was available online and published in the scientific journal ‘Vacunas’ entitled: ‘Menstrual abnormalities after COVID-19 vaccines: A systematic review’
A total of 78,138 vaccinated females were included in the reviews from 14 studies. It reported that ‘a significant number of women (52.05%) have experienced menstrual abnormalities after the COVID-19 vaccine.’
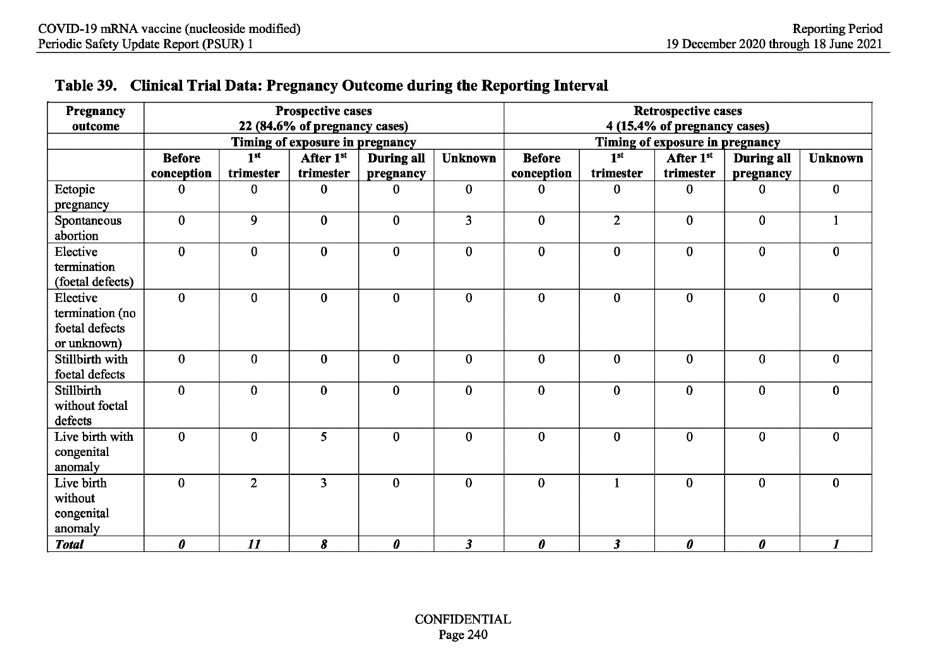
Pregnancy outcomes
One of the most alarming data sets I came across fell under this category. From Spring 2021, health authorities in most of the developed nations started to encourage pregnant and lactating women to get the novel mRNA vaccine. The damning data in the PSUR which was signed off by the summer of 2021, should have made those authorities do an abrupt U-turn but it didn’t.
Based upon the above request by the WHO, the MAH (BioNTech) looked at the outcome of the pregnancy cases observed in the clinical trial data. Table 39 below, shows 26 pregnancy outcomes from the 149 unique pregnancy cases, recorded during the reporting interval. Of those 26 outcomes, 15 resulted in spontaneous abortion (miscarriage) and 5 of the pregnancy outcomes resulted in live births with congenital anomalies.
In response to this alarming data, the report simply stated: ‘there was limited information regarding mother’s obstetric history which precluded meaningful causality assessment.’
Deaths
Turning to the data sets on deaths (fatal outcomes), below is a screenshot showing the most frequently reported causes of death from the post-authorization data.
During the reporting interval period, COVID-19 happened to be the number one cause of death (besides ‘Death’ itself)- the very disease that the vaccine was widely touted to protect against. Notice also how ‘sudden death’ was reported as one of the most frequent causes. Many reports in the mainstream media (post COVID-19 vaccine rollout) have covered stories of the young and healthy, suddenly dying, which is alarming.
Strangely, autopsy reports were only provided for 189 cases out of the 5042 cases from the post-authorisation data. See the results in the screenshot below.
Pulmonary embolism is caused by a thrombus that forms in a large vein, often in the legs, becomes detached and is carried by the bloodstream to the lungs, where it then blocks one of the arteries.. This was the most commonly reported disease in the autopsy results provided. What’s worth noting is that in July 2021, the US Food and Drug Administration linked the Pfizer-BioNTech vaccine to four new adverse events of interest (AEI), adding, however, there was no evidence of a causal link. Pulmonary embolism was among them, along with acute myocardial infarction (heart attack), immune thrombocytopenia (a blood disorder), and disseminated intravascular coagulation (a condition whereby blood clots form throughout the body).
According to the PSUR’s conclusion on deaths: ‘no new safety signals have emerged based on the review of these cases…safety surveillance will continue.’
With regards immune thrombocytopenia, the executive summary of the report stated:
It’s astounding how the first ever EU pharmacovigilance report for the Pfizer-BioNTech COVID-19 vaccine, loaded with such damning data, sailed through the European Medicines Agency’s radar, without raising any red flags but instead they went along with the conclusion, ‘the benefit-risk profile of BNT162b2 remains favourable.’ At that time, there was no long-term safety data for the EMA to rely on, however, the data which they did have in the short-term (known since the summer of 2021), should have sent the alarm bells ringing, only it didn’t, instead this experimental product was offered to younger age groups of the population and eventually to babies as young as 6 months of age.
Given the skyrocketing safety signals observed across various post-marketing vaccine surveillance programmes around the globe; the alarming findings in the Pfizer-BioNTech clinical trial documents; the leaked EMA assessment reports and internal emails, as well as the damning data found in PSUR #1- have served as a catalyst to urgently bring to light all other important pharmacovigilant reports.
Doctors and medical experts across Europe have now requested further reports with due process – more analysis will be rolled out as we gain access.
https://soniaelijah.substack.com/p/eu-safety-report-on-pfizer-biontech




No comments:
Post a Comment