The Demise of the AstraZeneca Jab
Another one bites the dust
The AstraZeneca’s COVID jab issue, a matter of grave concern, has been a topic I've been eager to address. The company's admission that its jab can cause blood clots, leading to its removal from the market, is a significant development. Our newest guest author, Jon Fleetwood, has provided an insightful piece on this matter for The Tenpenny Report. As I’m traveling this week and next, I’m sharing his article, which sheds light on this crucial issue, and adding a bit more information.
Share Dr. Tenpenny's Eye on the Evidence
+++++++++++++
AstraZeneca has faced a challenging year. In January, 35 individuals from the UK filed claims in the UK High Court, seeking over 80 million pounds (more than $10M USD) in compensation for complications they suffered from the AstraZeneca COVID-19 DNA vaccine. Fleetwood's analysis reveals that an additional 40 claims are expected, making this one of the most significant vaccine litigation cases in history, underscoring the gravity of the situation.
Fleetwood also reminds us that AstraZeneca is an official partner of the World Economic Forum (WEF) and has been funded by the Bill & Melinda Gates Foundation.
In April, AstraZeneca’s fate worsened as the company finally fessed up that its COVID jab can indeed cause blood clots, albeit, “in very rare cases.”
In an apparent about-turn that could pave the way for a multi-million-pound legal payout over the vaccine they developed with the University of Oxford, AstraZeneca admitted in court documents that its Covid vaccine can cause a rare side effect. More than 50 cases of a rare condition, thrombosis with thrombocytopenia syndrome (TTS), have been lodged in the High Court. TTS is characterized by blood clots and low levels of platelets following vaccination.
Shortly after the blood clot admission, the European Commission announced it was withdrawing the marketing authorization for AstraZeneca’s COVID-19 vaccine, called Vaxzevria, effective May 7, 2024. Interestingly, the company itself formally requested the withdrawal of its vaccine from the market.
The Data Tells the Story
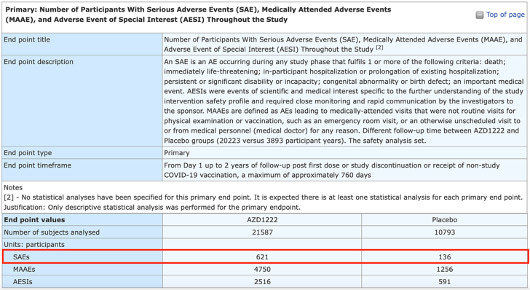
Clinical trial data submitted to the European Union Clinical Trials Register confirm that 1 in every 34 recipients of AstraZeneca Plc.’s COVID-19 injection has suffered a serious adverse event (SAE). The European Union (EU) clinical trial data also shows the vaccinated are more than twice as likely to suffer SAE than those who received a placebo. The troubling revelation comes after AstraZeneca withdrew the marketing authorization for its COVID shot in the European Union (EU).
AstraZeneca admitted in court that its COVID jab can cause thrombosis with thrombocytopenia syndrome (TTS). The new clinical trial documentation defines an SAE as fulfilling one or more of the following criteria:
Immediately life-threatening complications;
hospitalization or prolongation of existing hospitalization;
persistent or significant disability or incapacity;
congenital abnormality or birth defect;
an important medical event;
death.
The trial was a phase 3, double-blind, placebo-controlled, multicenter study conducted at 88 sites across the United States, Peru, and Chile. The trial lasted from August 2020 to March 2023 and enrolled 32,449 healthy or stable chronic medically condition participants aged 18 years or older. Participants were randomized 2:1 to receive either two doses of AstraZeneca’s AZD1222 vaccine or placebo (saline) given 4 weeks apart.The primary objectives were to evaluate the safety and efficacy of AZD1222 in preventing symptomatic COVID.
AstraZeneca sponsored the trial, which was funded by BARDA, the U.S. Department of Defense, the National Institute of Allergy and Infectious Diseases (NIAID), and the National Institutes of Health (NIH).
The data show that 621 out of 21,587 (one in 34) vaccine recipients suffered an SAE.
That’s compared to just 136 out of 10,793 (one in 79) in the placebo group, meaning the vaccinated were more than twice as likely to suffer a serious adverse event.
Screenshots from ClinicalTrialsRegister.eutaken May 28, 3024
The data also indicate that 4,750 (one in four) vaccine recipients suffered medically attended adverse events (MAAEs), defined as adverse events.
An MAAE is a medically-attended visit to a practitioner that was not planned and was not a routine visit for physical examination or for vaccinations. This includes an emergency room visit or an otherwise unscheduled visit to or from medical personnel (medical doctor) for any reason.
Moreover, one in eight vaccine recipients suffered adverse events of special interest (AESIs), defined as “events of scientific and medical interest specific to the further understanding of the study intervention safety profile and required close monitoring and rapid communication by the investigators to the sponsor.” That’s a mouthful. It means an adverse reaction, not often seen, that needs more investigation.
Only 591 (one in 18) in the placebo group experienced an AESI. (What was in the placebo? We have no idea what came through that needle.)
The clinical trial results were pointed out by Dr. David Cartland, a General Medical Council (GMC) registered practitioner in the U.K., who provided a link to the data in a Tuesday X (formerly Twitter) post.
“Needs to go viral!” Dr. Cartland wrote. “This is absolute proof of the vaccine failure. Astra Zeneca quietly submitted their Clinical Trail data as part of their application to have their license withdrawn.”
“No MSM outlet has reported on this,” he rightly pointed out.
Follow Jon Fleetwood on Instagram @realjonfleetwood / Twitter @JonMFleetwood
Where we are headed
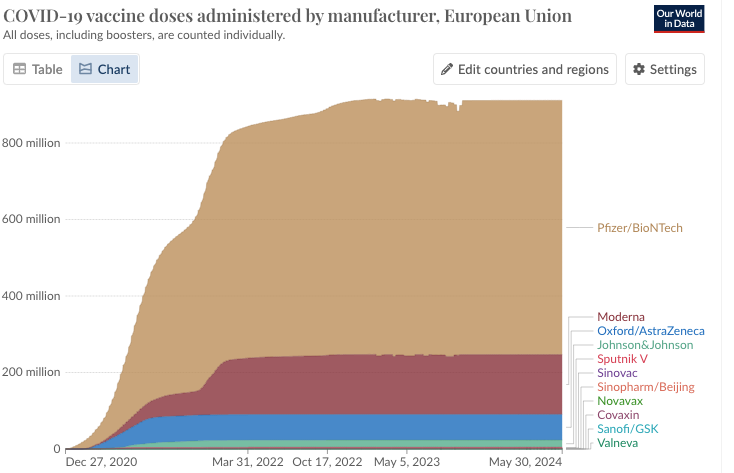
According to OurWorldData.org, which is updated each morning, with the most recent official numbers up to the previous day,
70.6% of the world population has received at least one dose of a COVID-19 vaccine.
13.58 billion doses have been administered globally, and 5,128 are now administered each day.
81.39% of people in the US have received at least one dose of the jab.
90.35% of people in Canada have received at least one dose of the jab.
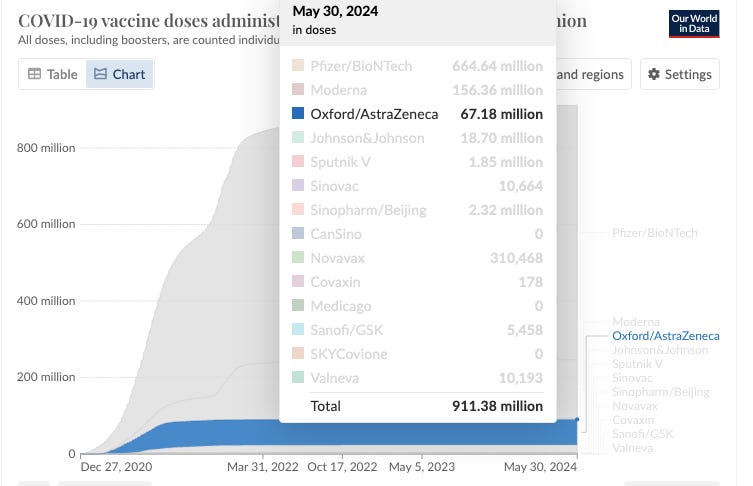
Of the 911.38Million doses administered, just over 67 Million were AstraZeneca jabs. Where does that leave those who have gotten a poisonous jab from a product that has been quietly removed from the market with little fanfare, and with a KNOWN track record for harm?
Will there be massive class-action lawsuits by those who were harmed, or those who will become sick in the future?
J&J COVID vaccines quietly removed too.
Almost a year ago to the day, Johnson and Johnson’s COVID-19 vaccine was pulled from the market, also for causing blood clots. The last remaining doses in the U.S. stockpile expired May 7, 2023, with the CDC now giving instructions to “[d]ispose of any remaining Janssen COVID-19 vaccine in accordance with local, state, and federal regulations.” Approximately 31.5 million doses had been delivered, with around 19 million of those administered to patients, according to CDC data.
Many people opted for this jab because it required fewer shots and was made on an adenovirus platform, similar to AstraZeneca jabs, instead of the mRNA platform of Pfizer and Moderna. The J&J injection was made from Ad26.COV-2.S shell, a human adenovirus first isolated from an anal specimen obtained from a 9-month-old male infant in 1956. The AstraZeneca vaccine used ChAdOx1, which is an adenovirus strain that normally infects chimpanzees.
Adenoviruses are highly inflammatory antigens. However, viral vaccines usually have not include them because adenoviruses are involved in tumorigenesis (create tumors) in animals and in cell culture.
Beyond blood clots, were these two experimental shots also responsible for the increasing number of “turbo cancers” we’re seeing?




No comments:
Post a Comment