Understanding And Dissolving Building Blocks Of Nanotechnology: Review Of Microplastic Polymers Found In Humans And Therapeutic Use of Essential Oils -Dr. Hildegard Staninger
After reviewing the recent article published in the ACS Journal "Detection of Various Microplastics in Patients Undergoing Cardiac Surgery", we now have a better understanding and confirmation as to what building blocks of hydrogel advanced nanomaterial plastics we need to address and disolve. The scientists found: PET: polyethylene terephthalate; PE: polyethylene; PU: polyurethane; PMMA: poly(methyl methacrylate); PA: polyamide; PP: polypropylene; PVC: polyvinyl chloride; PC: polycarbonate; PS: polystyrene.
This once again confirms what also Clifford Carnicom and I found in our Near Infrared spectroscopy findings of C19 vaccinated and unvaccinated blood. We found Polyamindes, polyethylene ( Polyenes) Polyvinyl plastics and Silicone.
Another recently published study from July 2023 analyzed blood clots.
Pigment microparticles and microplastics found in human thrombi based on Raman spectral evidence
In this study, eighty-seven environmental microparticles were detected and identified in thrombi with Raman Spectrometer. Among the microparticles identified, twenty-one phthalocyanine particles and one low-density polyethylene microplastic were definitely from synthetic materials. Phtalocyanine is used in medical nanotechnology for photodynamic treatments and imaging as well as chemical nanosensor with nonlinar optics and energy storage applications .
Hence, understanding from scientific research what the building block components of the polymer biosensing nanotechnology are, should guide our treatment approaches to help dissolve the building blocks needed for self assembly. I have previously described the metal binding capacity of EDTA to eliminate the metals in the body needed to build semiconducting microrobots and nanosensorsas well as the patent showing EDTA is capable of disolving hydrogels. In have recently shown that Methylene Blue has excellent, near magnetic binding capacity to hydrogel polymers, and hence can be used to eliminate these polymer plastics from the body. I also showed that Methylene Blue prevents the rubbery clot formation after letting 30 ml C19 unvaccinated blood sit overnight, while the untreated control developed the rubbery clot. Additionally, Methylene Blue could help with oxygen deprivation based on cyanide toxicity frompolymer breakdown molecules as discussed below.
The historical work of Dr Hildegard Staninger, a toxicology expert who for 40 years has studied the composition of the advanced nano materials deployed upon humanity and its treatment approaches. She was both an expert for Morgellon’s and Targeted Individuals and has done the most extensive chemical analysis on these advanced nanomaterials known in the world. This above published paper discusses the use of essential oil blends in dissolving Styrofoam plastics. Please read the history of this industry, for all the components now found as polymer microplastics in human tissues, are mentioned below. The toxic plastics have found their way into polymer nanotechnology components that are now found in the blood and tissue of human beings, fusing with their cells to create human Cyborgs. The reason why this paper is important, will become clear in future articles where I will deep dive into organic chemistry. The breakdown products of polyurethane creates cyanide groups that can bind oxygen and cause cellular asphyxiation. I will also show that genetic variability to degrade inert polyamide polymers can cause significant illness in the body, similar to Covid19 symptoms. Hence treatment will include detoxification strategies of cyanides, which includes methylcobalamin ( Vitamin B12) and Vitamin B2. Note that polystyrene that has been found in humans and the discussion of health effects that are exactly like “long Covid.” Please also note the data of cancer risk increase and adverse effects on pregnancy outcomes. There are people taking silica supposedly to treat the nanobots. Based on below information, I would recommend against that.
Below is a reproduction of her paper, with some minor exclusions due to length. The full article is attached below. I have highlighted sections particularly relevant to the nanotechnology polymers.
INTRODUCTION
Quoting from Dow’s Building Solutions Media Resources web site, “There’s No Such thing as a Styrofoam™ cup.” Styrofoam™ Brand Foam is a registered trademark of the Dow Chemical Company and has been a valued asset of Dow Chemical for more than 60 years.
The Styrofoam™ Brand name is often misused as a generic term for disposable foam products such as coffee cups, cooler and packaging materials. These materials, however, are made from expanded polystyrene (also known as EPS) and do not have the insulation value, compressive e strength or moisture-resistant properties of Styrofoam™ Brand Extruded Polystyrene Insulation products as represented by the Dow Chemical Company’s Registered Trademarked products.
Over the last twenty years a new breed of products have been developed by academia, agriculture, governmental agencies, pharmaceutical and industry by incorporating seeded-emulsion polymerization of styrene with waterborne polyurethane stabilizers in many new technologies such as:
1. Microbeads, nanospheres, pebbles, liquid viral crystals, and gems in biological pesticide technology, nano drug delivery systems, ocular gene therapy and chitosan nanoparticles as gene therapy vector via gastrointestinal mucosa.
2. Mannosylated chitosan nanoparticle-based cytokine gene therapy for cancer treatment, gene transfer using self-assembled ternary complexes of cationic magnetic nanoparticles, plasmid DNA and cell-penetrating Tat peptides.
3. Nano bio sensors for intelligent food packaging, pH dependent silicon nanospheres and nano tubes, and reinforcement of folate absorption using nano technology.
History of Polyurethane, Styrene, Waterborne Polyurethane,
Konjac Glucomannan and Modified Food Starch
Polyurethane
Polyurethane (PUR) is any polymer consisting of a chain of organic units joined by urethane (carbamate) links. Polyurethane polymers are formed through step-growth polymerization by reacting a monomer containing at least two hydroxyl (alcohol) groups in the presence of a catalyst.
The pioneering work on polyurethane polymers was conducted by Otto Bayer and his coworkers in 1937 at the laboratories of I.G. Farben in Leverkusen, Germany. They recognized that using the polyaddition principle to produce polyurethanes from liquid diisocyantes and liquid polyether or polyester diols seemed to point to special opportunities, especially when compared to already existing plastics that were made by polymerizing olefins, or by polycondensation. New Monomer combination also circumvented existing patents obtained by Wallace Carothers on polyesters. Initially, work focused on the production of fibres and flexible foams. With development constrained by World War II (when Pus were applied on a limited scale as aircraft coating was not until 1952 that polyisocyantes became commercially available. Commercial production of flexible polyurethane foam began in 1954, based on toluene diisocyanate (TDI) and polyester polyols. The invention of these foams (initially called imitation Swiss cheese by the inventors) was thanks to water accidentally introduced in the reaction mix. These materials were also used to produce rigid foams, gum rubber, and elastomers. Linear fibres were produce from hexamethylene diisocyanate (HDI) and 1,4-butaneediol (BDO).
The first commercially available polyether polyol, poly (tetramethylene ether) glycol, was introduced by DuPont in 1956 by polymerizing tetrahydrofuran. Less expensive polyalkylene glycols were introduced by BASF and Dow Chemical the following year, 1957. These polyether polyols offered technical and commercial advantages such as low cost, ease of handling, and better hydrolytic stability; and quickly supplanted polyester polyols in the manufacture of polyurethane goods. Other PU pioneers were Union Carbide and the Mobay corporation, a U.S. Monsanto/Bayer joint venture. In 1960 more than 45,000 tons of flexible polyurethane foams were produced. As the decade progressed, the availability of chlorofluoralkane blowing agents, inexpensive polyester polyols, and methylene diphenyl diisocyante (MDI) heralded the development and use of polyurethane rigid foams high performance insulation materials. Rigid foams based on polymeric MDI (PMDI) offered better thermal stability and combustion characteristics than those based on TDI. In 1967 urethane modified polyisocyanurate rigid foams were introduced, offering even better thermal stability and flammability resistance to low-density insulation products. Also during the 1960’s, automotive interior safety components such as instrument and door panels were produced by back-filling thermoplastic skins with semi-rigid foam.
In 1969, Bayer AG exhibited an all plastic car in Dusseldorf, Germany. The plastic car involved Polyurethane Reaction Injection Molding technology, which allowed for parts to be molded. In 1983, the first plastic-body automobile was built in the United States, the Pontiac Fiero. In the early 1990’s, because of these simple volatile chemicals yielding an impact on ozone depletion, the Montreal Protocol lead to the greatly reduced use of many chlorine-containing blowing agents, such as trichlorofluoromethane (CFC-11). Other haloalkanes, such as hydrochlorofluorocarbon 1,1-dichloro-1-fluoroethane ((HCFC-141b), were used as interim replacements until their phase out under the IPPC directive on greenhouse gases in 1994. Between the 1990’s to present day the advent of nano technology allowed these same compounds to be utilized in the manufacture of nanospheres, nano carbon tubes, single walled nano tubes, multiwalled nano tubes, nano belts, claws, arm chairs, and hooks to name just a few. Along with the time released or pH controlled release of specific nano delivery systems to be utilized in the pharmaceutical and medicine under the term “nanomedicine” and “gene delivery systems.” No matter, what it is called a plastic polyurethane polymer is still a hazardous material.
Polyurethane formulations cover an extremely wide range of stiffness, hardness, and densities. These materials include:
· Low-density flexible foam used in upholstery, bedding, and automotive truck seating.
· Low-density rigid foam used for thermal insulation and RTM cores.
· Soft solid elastomers used for gel pads and print roller.
· Hard solid plastics used as electronic instrument bezels and structural parts.
Polyurethane products are often called “urethanes.” They should not be confused with specific substance urethane, also known as ethyl carbamate. Polyurethanes are neither produced from ethyl carbamate, nor do they contain it.12 Pesticides that are long lasting and cause acetyl cholinesterase inhibition are termed carbamates and organophosphates. They are usually composed of methylcarbamates or ethylcarbamate.13
Styrene
Styrene is primarily used in the production of polystyrene plastics and resins. Acute (short- term) exposure to styrene in humans results in mucous membrane and eye irritation, and gastrointestinal effects. Chronic (long-term) exposure to styrene in humans results in effects on the central nervous system (CNS), such as headache, fatigue, weakness, and depression, CSN dysfunction, hearing loss, and peripheral neuropathy. Human studies are inconclusive on the reproductive and developmental effects of styrene; several studies did not report an increase in developmental effects in women who worked in the plastics industry, while an increased frequency of spontaneous abortions and decreased frequency of births were reported in another study. Several epidemiologic studies suggest there may be an association between styrene exposure and an increased risk of leukemia and lymphoma. However, the evidence is inconclusive due to confounding factors. EPA has not given a formal carcinogen classification to styrene.
Uses
· Styrene is used predominately in the production of polystyrene plastics and resins. Styrene is also used as an intermediate in the synthesis of materials used for ion exchange resins and to produce copolymers.
Sources and Potential Exposure
· Indoor air is the principal route of styrene exposure for the general population. Average indoor air levels of styrene are in the range of 1 to 9 µg/m3, attributable to emissions from building materials, consumer products, and tobacco smoke.
· Ambient air in urban locations contains styrene at average concentrations of .29 to 3.8 µg/m3, while styrene in rural and suburban air has been measured at 0.28 to 0.34 µg/m3.
· Occupational exposure to styrene occurs in the reinforced plastics industry and polystyrene factories.
Assessing Personal Exposure
· Laboratory tests can determine styrene by measuring the breakdown products in the urine. However, these tests are only useful for detecting very recent exposures.
Health Hazard Information
Acute Effects
· Acute exposure to styrene in humans results in respiratory effects, such as mucous membrane irritation, eye irritation, and gastrointestinal effects.
· Tests involving acute exposure of rats and mice have shown styrene to have low to moderate toxicity by inhalation and oral exposure.
Chronic Effects (Noncancerous)
· Chronic exposure to styrene in humans results in effects on the CNS, with symptoms such as headache, fatigue, weakness, depression, CNS dysfunction (reaction time, memory, visuomotor speed and accuracy, intellectual function), and hearing loss, peripheral neuropathy, minor effects on some kidney enzyme functions and on the blood.
· Animal studies have reported effects on the CNS, liver, kidney, and eye and nasal irritation from inhalation exposure to styrene.
· Liver, blood, kidney, and stomach effects have been observed in animals following chronic oral exposure.
· The Reference Concentration (RfC) for styrene is 1 milligram per cubic meter (mg/m3) based on CNS effects in occupationally exposed workers. The RfC is an estimate (with uncertainty spanning perhaps an order of magnitude) of a continuous inhalation exposure to the human population (including sensitive subgroups) that is likely to be without appreciable risk of deleterious noncancerous effects during a lifetime. It is not a direct estimator of risk but rather a reference point to gauge the potential effects. At exposures increasingly greater than the RfC, the potential for adverse health effects increases. Lifetime exposure above the RfC does not imply that an adverse health effect would necessarily occur.
· EPA has medium confidence in the study on which the RfC was based because, although the study documents concentration-response relationships of CNS effects in a relatively small worker population, the results are consistent with a number of other studies showing central effects in chronically exposed worker populations; medium to high confidence in the database because the chronic laboratory animal studies addressing noncancerous endpoints were not available, although a number of human exposure studies support the choice of critical effect; and, consequently, medium confidence in the RfC.
· The Reference Dose (RfD) for styrene is 0.2 milligrams per kilogram body weight per day (mg/kg/d) based on red blood cell and liver effects in dogs.
· EPA has medium confidence in the principal study on which the RfD was based because it was well done and the effect levels seem reasonable, but the small number of animals/sex/dose prevents a higher confidence; medium confidence in the database because it offers strong support, but lacks a bona fide full-term chronic study; and, consequently, medium confidence in the RfD.
Reproductive/Developmental Effects
· Human studies have not reported an increase in developmental effects in women who worked in the plastics industry, while an increased frequency of spontaneous abortions and a decreased frequency of births were reported in a study on the reproductive effects of styrene in humans. However, these studies are not conclusive, due to the lack of exposure data and confounding factors.
· Animal studies have not reported developmental or reproductive effects from inhalation exposure to styrene.
· Lung tumors have been observed in the offspring of orally exposed mice.
Cancer Risk
· Several epidemiologic studies suggest that there may be an association between styrene exposure and an increased risk of leukemia and lymphoma. However, the evidence is inconclusive due to multiple chemical exposures and inadequate information on the levels and duration of exposure.
· Animal cancer studies have produced variable results and provide limited evidence for carcinogenicity.
· IARC has classified styrene as a Group 2B, possibly carcinogenic to humans.
· Styrene oxide is a reactive metabolite of styrene and shows positive carcinogenic results in oral exposure bioassays. Styrene oxide has been detected in workers exposed to styrene. IARC has classified this metabolite as a Group 2A, probable human carcinogen.
· EPA does not have a carcinogen classification for styrene; the chemical currently is undergoing an EPA Integrated Risk Information System (IRIS) review to establish such a classification.
Waterborne Polyurethane
Waterborne Polyurethanes are fully reacted Urethane polymers dispersed in water. These products contain no residual free isocyanate. They are formulated the same way as polyurethanes, but do not use isocyanates as a catalyst. In many waterborne and regular polyurethanes organometallic compounds based on mercury, lead, tin (dibutyltin dilaurate), bismuth (bismuth octanoate), and zinc are used as polyurethane catalysts. Mercury carboxylates, such as phenylmercuric neodeconate, are particularly effective catalysts for polyurethane elastomer, coating, and sealant applications, since they are very highly selective towards the polyol+isocyanate reaction. Mercury catalyst can be used at low levels to give systems a long pot life while still giving excellent back-end cure. Lead catalysts are used in highly reactive rigid spray foam insulation applications, since they maintain their potency in low –temperature and high-humidity conditions. Due to their toxicity and the necessity to dispose of mercury and lead catalysts and catalyzed material as hazardous waste in the United States, formulators have been searching for suitable replacements. Since the 1990’s, bismuth and zinc carboxylates have been used as alternatives but have short comings of their own. In elastomer applications, long pot life systems do not build green strength as fast as mercury catalyzed systems. In spray foam applications,
bismuth and zinc do not drive the front end fast enough in cold weather conditions and must be otherwise augmented to replace lead. Alkyl tin carboxylates, oxides, and mercaptides oxides are used in all types of polyurethane applications. For example, dibutyltin dilaurate is a standard catalyst for polyurethane adhesives and sealants, dioctyltine mercaptide is used in microcellular elastomer applications, and dibutyltin oxide is used in polyurethane paint and coatings, nano enhanced coatings, anti-microbic agent for nano particles/wires and bioengineering applications of DNA/RNA gene therapy.Tin mercaptides are used in formulations that contain water, as tin carboxylates are susceptible to degradation from hydrolysis.
Konjac Glucomannan
Konjac Glucomannan is a water-soluble polysaccharide that is considered a dietary fiber. Glucomannan is a food additive used as an emulsifier and thickener. Products containing glucomannan, marketed under a variety of brand names, are also sold as nutritional supplements for constipation, obesity, high cholesterol, acne vulgaris and type 2 diabetes. Though there is some clinical support for potential health benefits, the U.S. Food and Drug Administration (FDA) has not approved any product containing glucomannan for the treatment of these medical conditions. Several companies selling products containing glucomannan have been disciplined by the U.S. Federal Trade Commission (FTC) for misleading or exaggerated claims pertaining to the health benefits of glucomannan.
Glucomannan is mainly a straight-chain polymer, with a small amount of branching. The component sugars are β-(1→4)-linked D-mannose and D-glucose in a ratio of 1.6:1.23The degree of branching is about 8% through β-(1→6)-glucosyl linkages.
Glucomannan comprises 40% by dry weight of the roots or corm of the konjac plant. It is also a hemicellulose that is present in large amounts in the wood of conifers and in smaller amounts in the wood of dicotyledons. Clinical and pre-clinical studies have shown several potential health benefits of glucomannan. Konjac glucomannan, waterborne urethane/polystyrene and modified food starch has been used in many forms of thin film nano lithography and other related technologies in creating nano composites and Sol-gel integrated technologies.
Glucomannan is a soluble fiber, and as such, has been investigated for the treatment of constipation. Glucomannan may relieve constipation by decreasing fecal transit time. In the treatment of chronic constipation, glucomannan significantly improved symptoms of constipation while being well-tolerated and free of relevant side effects.
Obesity
Clinical evidence suggests glucomannan may be beneficial in weight loss.28 Because it is a soluble fiber, it absorbs water to form a viscous gel-like mass. This mass may promote feelings of satiety while traveling through the gastrointestinal tract. In obese patients, taking 1 gram of glucomannan with 8 ounces (250 ml) of water 1 hour before each of 3 meals daily over 8 weeks resulted in an average weight loss of 5.5 pounds (2.5 kg).
Cholesterol and other Lipids
Glucomannan has demonstrated statistically significant improvements in the total cholesterol of obese patients.29 In healthy men, 4 weeks of taking 3.9 grams of glucomannan decreased total cholesterol, low-density lipoprotein, triglycerides, and systolic blood pressure; notably, triglycerides dropped by 23%.30 Glucomannan has also been tested in children with high cholesterol in conjunction with a diet. Interestingly, greater decreases in total cholesterol and low-density lipoprotein were observed in female children when compared to male children.31 When used in conjunction with chitosan, glucomannan decreases serum cholesterol possibly by increasing steroid excretion via the feces.32
Type 2 Diabetes
Glucomannan may be useful as a therapeutic adjunct for type 2 diabetes. It has been shown to improve the lipid profile and alleviate the fasting blood glucose levels of type 2 diabetics.33
Commercial Use
As a food additive, glucomannan is used as an emulsifier and thickener. It has E number E425(ii). Glucomannan also makes up the majority of shirataki noodles and an ingredient in modified food starch with waterborne polyurethane nano delivery systems for time released materials for internal consumption.
In 2002, a number of products containing konjac-derived glucomannan were recalled as choking hazards.34 Innovative plasticized starch films modified with water borne polyrethane from renewable sources were developed using rapseed oil and then modifying the glycerol plasticized starch to develop water resistant materials were developed by scientists at the university of Reims, France.
Modified Food Starch
Modified starch is a food additive which is prepared by treating starch or starch granules, causing the starch to be partially degraded. Modified starch is used as a thickening agent, stabilizer, or an emulsifier. Apart from food products, modified starch is also found in pharmaceuticals, paper and many other applications.
Starches are modified for a number of reasons. Starches may be modified to increase their stability against excessive heat, acid, shear, time and cooling or freezing; to change their texture; to decrease the viscosity, or to lengthen or shorten gelatinization time.
A modified starch may be an instant starch which thickens and gels without heat, or a cook- up starch which must be cooked like regular starch.
Acid-treated starch (E1401), usually simply called "modified starch", is prepared by treating starch or starch granules with inorganic acids, breaking down the starch molecule and thus reducing the viscosity.
Other treatments may produce modified starch with different E numbers, such as alkaline- modified starch (E1402), bleached starch (E1403), oxidized starch (E1404), enzyme-treated starch (INS: 1405), acetylated starch (E1420), and acetylated oxidized starch (E1451).
Pre-gelatinized starch is used to thicken instant desserts, allowing the food to thicken with the addition of cold water or milk. Similarly, cheese sauce granules (such as in Macaroni and Cheese or lasagna) or gravy granules may be thickened with boiling water without the product going lumpy. Commercial pizza toppings containing modified starch will thicken when heated in the oven, keeping them on top of the pizza, and then become runny when cooled.
A suitably-modified starch is used, quite successfully (with respect to the taste), as a fat substitute for low-fat versions of traditionally fatty foods, e.g., reduced-fat hard salami having about 1/3rd the usual fat content. For such uses, it is an alternative to the product Olestra.
Modified starch is added to frozen products to prevent them from dripping when defrosted. Modified starch, bonded with phosphate, allows the starch to absorb more water and keeps the ingredients together. Modified starch acts as an emulsifier for French dressing by enveloping oil droplets and suspending them in the water. Acid-treated starch forms the shell of jelly beans. Oxidized starch increases the stickiness of batter.
Genetically Modified Starch
Modified starch should not be confused with genetically modified starch, which refers to starch from genetically engineered plants, which have been genetically modified to produce novel carbohydrates which might not naturally occur in the plant species being harvested. The modification in this sense refers to the genetic engineering of the plant DNA, and not the later processing or treatment of the starch or starch granules.
Genetically modified starch is of interest in the manufacture of biodegradable polymers and noncellulose feedstock in the paper industry, as well as the creation of new food additives such as the following: Dextrin (E1400), Starch gelatinization, Retrogradation (starch), Resistant starch, glucose syrup and glucose. High Fructose Corn Syrup that has been made with genetically modified corn may be in this same category if made into corn starch.
Waterborne Polyurethane, Nanosilica and Modified Starch
The field of nanotechnology has grown in the last three years from 1.7 million papers on the subject to over 75 million papers, which translates into the industrialization of applied nanotechnologies. Nanosilica in the form of nano tubes, belts, gels, and special architectured advanced nano materials may become the new leader as compared to carbon nano tubes in the field of nanomedicine and gene therapy. Nano-SiO2has been used in experiments at the College of Chemistry, Central China Normal University, Wuhan, People’s Republic of China to modify waterborne polyurethane. The morphology and performance of the waterborne polyurethane in these tests showed that nano-SiO2 could be evenly dispersed in the waterborne polyurethane and had a better resistance to high temperature and water. Properties and structure tests were performed by Fourier transform infrared spectra (IR) and other analytical methods.36
In 2006, Fang Zheng, et.al., wrote a paper on the use of chitosan nanoparticle as a gene therapy vector via gastrointestinal mucosa administration using three different types of vectors with a green fluorescent protein. The materials to create the encapsulated plasmid DNA (pDNA) for gene therapy utilizes waterborne polyurethanes and further encapsulation with modified starches. The green fluorescent protein (GFP) was found in the mucosa of the stomach and duodenum, jejunum, ileum and large intestine in 100%, 88.9%, 77.8%, and 66.7% of the nude mice in the experiment with an optimal chitosan/pDNA being 3:2:1.37
In 2007 to present date in California aerial spraying has occurred for the Brown Moth, West Nile Virus, and many other vectors that come under the Chemical and Biological Warfare Act of 1949, US EPA, and other governmental agencies. The spraying of the Brown Moth utilized a pesticide known a Check Mate, which was a new biological pesticide using pheromone technology on micro beads or nanospheres. These materials were sprayed over specific areas of the State of California. After the spraying of these materials a yellow fluorescent dye was found in the sclera of exposed individuals eyes.38 The micro beads were made of polyurethanes, silicon, polystyrene and other plastic polymer material at a specific diameter size due to various criteria set by its manufacturer or manufacturer’s client.
Essential Oils the Missing Link in Polystyrene Cellular Exposures
Plants not only play a vital role in the ecological balance of our planet, but they have also been intimately linked to the physical, emotional, and spiritual well being of people since the beginning of time.39
The plant kingdom continues to be the subject of an enormous amount of research and discovery. At least 30 percent of prescription drugs in the United States are based on naturally occurring compounds from plants. Each year, millions of dollars are allocated to universities searching for new therapeutic agents that lie undiscovered in the bark, roots, flowers, seeds, and foliage of jungle canopies, river bottoms, forests, hillsides, and vast wilderness regions throughout the world.40
The primary areas of the plant, which contains the most powerful ingredient in therapeutic essential oils and plant extracts, have been a universal tapestry of history since the beginning of time, when she was in love with eternity.41 Essential oils are aromatic volatile liquids distilled from shrubs, flowers, trees, roots, bushes, and seeds.
The chemistry of essential oils is very complex: each one may consist of hundreds of different and unique chemical compounds. Moreover, essential oils are highly concentrated and far more potent than dried herbs. The distillation process is what makes essential oils so concentrates. It often requires an entire pliant or more to produce a single drop of distilled essential oil.42, 43
Essential oils are substances that definitely deserve the respect of proper education. Users need to be fully versed in the chemistry and safety of the oils. However, this knowledge is not being taught at universities in the United States and that is why many integrated medical programs and essential oil manufacturers have created continuing education and certification programs. In the United States there is a lack of institutional information, knowledge, and training on essential oils and the scientific approach to aromatherapy.
The American Chemical Society, since its inception as Charted by the U.S. Congress in 1876 has been the premier membership organization of chemists, chemical engineers, and allied professionals worldwide. This was the original organization that offered to chemist a special training course that was used to identify chemicals by smell, which was adapted early on in the National Institute of Occupational Health and Safety Institute and the Occupational Safety and Health Administration for chemical identity through smell.43, 44, 45
Many essential oils and their oil blends have been used as an anti-microbial agent as seen in the Middle Ages, when a blend of oils commonly known in modern times as Thieves® by Young Living Essential Oils® was used by gypsies and other individuals to guard against infection from exposure to the Bubonic Plague.46 During World War II, Melaleuae alternifolia (Tea Tree Oil/melaleuca) was found to have very strong antibacterial properties and worked well in preventing infection in, open wounds.47
Other essential oil blends, such as Inner Solution containing juniper, fennel, mugwart, germanium, lavender, orange, peppermint, capsicum extract, jojoba and cinnamon are mixtures of oils and extracts that have been known as an ancient Euro-Asian recipe dating back to the days of Marco Polo when he traveled the Silk Road Trail. Inner Solution addresses specifically the metabolism of fat as it breaks up the actual cause of the fat build- up in the cells and systemic organs of the body.48 Many times individuals experience weight gain, severe inflammation, loss of breath, and increased cholesterol. These symptoms may be the buildup of hazardous materials and hormones in the localized areas of the body that stimulate the production of cholesterol and triglycerides that lead to the manufacture of fat. The manufacturer of fat is the body’s natural way of protecting and encapsulating the toxic substances in the body so they do not destroy nerves or deplete minerals and vitamins which may stimulate the initiation of disease. The use of waterborne polyurethane with modified food starches as biological pesticide inert ingredients, food additives, nano drug delivery systems, and DNA gene may be the real cause of the United States obesity problems.49, 50
Polystyrene and Essential Oils or Oil Blends
The essential oils of Thieves® and Inner SolutionTM contain the oils that have been used for respiratory distress, nasal infections, and other types of infections for over 800 years or more an experiment was designed to address internal exposure to modified food starch with waterborne polyurethane or chitosan and waterborne polyurethane/styrene (ingestion), inhalation of starch incorporated nanospheres, nano gems, microspheres or pebbles that are used in weather modification and security sensing throughout the United States and abroad.51, 52
The Experiment
The following is a description of as simple experiment utilizing a total of three (3) polystryrene foam cups (6 ounce size). Each cup contained 4 ounces of Los Angeles, California tap water. The experiment was designed to determine the rate of time it would take to melt the polyurethane-styrene cup foam. These same materials are used in making many advanced nano materials as previously discussed in this report. The contents of the cups were as follows:
Cup No. 1 was the CONTROL, which had nothing added to it except the tap water. Cup No. 2 contained Tap water with 6 drops of INNER SOLUTIONTM.
Cup No. 3 contained Tap water with 6 drops of THIEVES®.
The cups were observed for visual observations over time. See Table 2. By referring to Table 2, one can observe that NO reaction occurred in the Control Cup, while the Inner SolutionTMcup had melted/etched through the polyurethane-styrene foam cup within 11 minutes and 36 seconds.
The Thieves® containing cup etched through the cup, leaving a distinct ring on the inner side of the cup, but did not completely melt or etch through the cup in 11 minutes and 36 time. The conclusion of the experiment was when the reaction time for melting/etching the polyurethane-styrene foam occurred. See Photograph 1: Control Cup; Photograph 2:
Inner Solution™ Cup; and Photograph 3: Thieves® Cup.
Referring back to Photographs 1 – 3 or the respective cups noting the times showing the comparative reactions as stated in Table 2, one will observe the melting away of polyurethane-styrene. This experiment was conducted to show the possible applications of therapeutic grade essential oils and oil blends to aid in dissolving the build-up residue upon cell membranes and organ system tissues from internal exposure to the accumulative effects of waterborne polyurethane mixed with modified food starch.
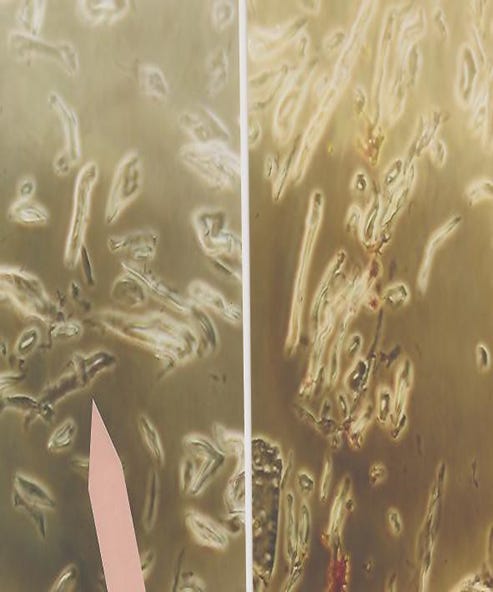
Waterborne polyurethane-styrene with modified food starch has been used to make nanospheres and some forms of pebbles or gems. These later nanotechnology materials are used in the production of materials for weather modification and location intelligence surveillance. Nano pebbles and gems are the new breed of smart dust and smart crystal motes.52 Mankind is facing the Brave New World53 of the commercialization of these materials into their everyday life without the advanced nano materials being tested for health and safety concerns. The US EPA has scheduled their outlined criteria for risk assessment guidelines for environmental concerns of exposure and utilization of advanced nano materials to be released in 2013, but what is happening NOW to the individuals and the environment. Exposure to plastic vapors and its degradation products in the work place has been recognized as a highly hazardous work environment, when engineering controls and other measures are not utilized to protect the workers. The advent of advanced nano technologies that can be absorbed into the skin by determine its internal function by the layers of dispersion through various pH’s within the human body will be of great concerns for the individuals that do not protect themselves from internal residual exposures. See Photograph 4 – Nanospheres housing Chinese lantern silicon nano tubes, note adjacent red blood cells human.
Photograph of Nanospheres housing Chinese lantern silicon Nanotubes; note adjacent human red blood cell and nanotechnology Comparative designs per Dr. Wang, Georgia Institute of Technology.
A) Silicon Nanospheres in Human Blood with various dye coatings with Chinese Lantern Nanotubes in Human Blood. Taken from Project: Fiber, Morgellons & Meteorite. © 2006 H. Staninger. Photomicrograph taken by Dr. Rahim Karjoo, Pathologist.
Looking at Photograph 5 – Human, Caucasian female Upper skin surface biopsy showing green nano horns on its surface. The nano horns identified were composed of polyurethane-styrene. The photographs confirm exposure to polyurethane-styrene based nano technology with its core containing silicon nano tubes called Chinese laterns (Photograph 4). The silicon nano tubes may be loaded with a gene therapy of DNA plasmids, siRNA, HELA cells, or many other substances like virons or vaccines to name a few. These types of bio or nano technologies are the currently the new nanomedicine and gene therapy pharmaceuticals be used by the medical community with Medicare reimbursement as they replace the petroleum based pharmaceuticals.56, 57
CONCLUSION
The current use of advance nano materials in pharmaceuticals, pathogen countermeasures, drug delivery systems, coatings, vaccine actuators, DNA/RNA vaccines, viral protein envelopes, adenoviral delivery systems, anti-microbial agents, artificial blood and many other areas without the comprehensive evaluation process as performed on standard chemical categories by our governmental regulators, mankind and the environmental will be see diseases as they never had before at an accelerated rate. A prime example being the use of nano chrysotile asbestos particles with nano dyes to determine specific tumors within the lungs and stomach lining of humans. The smaller the size of an asbestos fiber, will only allow its penetration into the cell membranes at an accelerated rate, especially if the individual has been exposed to the contaminant of a polio vaccine – Simian Virus 40 mutation.55 Many of the universities that make the advanced nano materials, such as Sencil™ does not have an appropriate Material Safety Data Sheet upon healthcare providers request as required under US OSHA General Industry Standard 1910.1200 Hazard Communication Standard (Right to Know).
The General Industry Standard 1910.1200 commonly known as the Right to Know standard, specifically states that the employee has the right to know if the chemicals they are working with in the workplace are hazardous substances as such as carcinogens, skin sensitizer, reproductive toxin, physical hazard, poison (class A or B), radiation, oxidizers, noise, and other similar categories of exposures that are associated with occupational diseases.
Currently, industry addresses these chemicals through training and engineering controls, but these same substances are being used in the manufacture of advanced nano materials for nanotechnology. The smaller the size of the molecule laced with hazardous substances and materials the greater the risk to health. The routes for exposure being inhalation, absorption and ingestion are still the same. The only factor being that the smaller the agent material the greater the ability for it to get into the cell membrane, cytoplasma and through the nuclear membrane and do its damage or repair in the case of gene therapy without the immune system responding.
Exposure to advanced nano materials will be the new challenges for industrial hygienist, environmentalist, safety engineers, health care provider and many others, in the decades to come. All of these and other professionals will have to approach the hazardous effects upon the human body and the environment with a mind. This type of mind must be able to accept the reality of the cybordization of mankind and environment from its initial inception of exposure to advanced materials such as Smart Dust.58 The time is NOW to address these concerns not the years to come. Already the mass population of the world is already experiencing plastic growing from their own bodies as a two part polyester resin with a silicon head 59, 60 under the ICD-Code of C17.800.518 – Morgellons Disease or Fiber Disease, which should really be called by its real name category – “Toxicological Effects from Exposure to Advanced Nano Materials.” The use of essential oils and oil blends may be the only therapeutic means for individuals and professional health care providers to remove advanced materials, such as waterborne polystryrene based nano spheres that bind to nasal and lung celluar membranes as they reduce cellular respiration levels of oxygen in other organ systems. A simple 800 plus year remedy and effective non-invasive modality to be used, when conventional therapeutic modalities do not address exposure to advanced nano materials that are composed of mixtures of polyurethane and styrene composite materials.
Here is the full paper, please find additional tables and information in it:
Ieia Nanomodifiedfoodstarchpaper Complete 3 26 12 (1)
1.16MB ∙ PDF file
Final comments:
The above test shows dissolution of polystyrene, which was also found by the previously named study in human cardiac tissue. The protocol for detoxification based on below findings are the ingredients of Inner Solution ( Grapefruit oil, lemon oil and Cinnamon oil 2 drops each in a veggie caps taken daily.) Inner Solutions is no longer available, so I recommend people make their own capules, 2 drops of each oil taken daily. Thieves is available. Additionally, Oregano Oil and Peppermint Oil capsules are also used for the detoxification of polymer plastics from the body. I am doing testing on vaccinated deceased blood clots to assess if these can be disolved after embalming. I also am doing several other tests with these essential oils in regards to live blood analysis. Given that they show activity against some of the plastics that are found in humans, this is a promising addition to our treatment arsenal. Further studies to corroborate effectiveness are underway. I wanted to share this important historical paper that has been backed up by decades of successful treatment of advanced nanmaterials warfare ( Morgellons) and Targeted Individual victims by the Author, who is a world expert in toxicology and treatment of advanced nanomaterials.




No comments:
Post a Comment